Advertisements
Advertisements
Question
Can absolute electrode potential of an electrode be measured?
Solution
No. It cannot be measured. We can only measure the difference in electrode potential between the two half-cells. We can also measure electrode potential difference concerning a standard electrode.
APPEARS IN
RELATED QUESTIONS
What happens if external potential applied becomes greater than E°cell of electrochemical cell?
Among Zn and Cu, which would occur more readily in nature as metal and which as an ion?
A solution of CuSO4 is electrolysed using a current of 1.5 amperes for 10 minutes. What mass of Cu is deposited at cathode? [Atomic mass of Cu = 63.7]
A current strength of 3.86 A was passed through molten Calcium oxide for 41minutes and 40 seconds. The mass of Calcium in grams deposited at the cathode is (atomic mass of Ca is 40g/mol and 1F = 96500 C).
Cell equation: \[\ce{A + 2B^- -> A^{2+} + 2B}\]
\[\ce{A^{2+} + 2e^- -> A}\] E0 = +0.34 V and log10 k = 15.6 at 300 K for cell reactions find E0 for \[\ce{B^+ + e^- -> B}\]
Can Fe3+ oxidises bromide to bromine under standard conditions?
Given: \[\ce{E^0_{{Fe^{3+}|Fe^{2+}}}}\] = 0.771 V
\[\ce{E^0_{{Br_{2}|Br^-}}}\] = −1.09 V
If the half-cell reaction A + e– → A– has a large negative reduction potential, it follow that:-
If 0.5 amp current is passed through acidified silver nitrate then in 100 minutes the mass of silver, deposite on cathode is (eq. wt. of silver nitrate + 108).
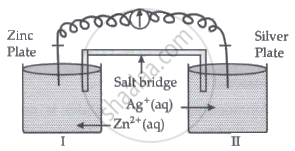
Read the passage given below and answer the questions that follow:
|
Oxidation-reduction reactions are commonly known as redox reactions. They involve transfer of electrons from one species to another. In a spontaneous reaction, energy is released which can be used to do useful work. The reaction is split into two half-reactions. Two different containers are used and a wire is used to drive the electrons from one side to the other and a Voltaic/Galvanic cell is created. It is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A salt bridge also connects to the half-cells. The reading of the voltmeter gives the cell voltage or cell potential or electromotive force. If \[\ce{E^0_{cell}}\] is positive the reaction is spontaneous and if it is negative the reaction is non-spontaneous and is referred to as electrolytic cell. Electrolysis refers to the decomposition of a substance by an electric current. One mole of electric charge when passed through a cell will discharge half a mole of a divalent metal ion such as Cu2+. This was first formulated by Faraday in the form of laws of electrolysis.
|
- Is silver plate the anode or cathode? (1)
- What will happen if the salt bridge is removed? (1)
- When does electrochemical cell behaves like an electrolytic cell? (1)
- (i) What will happen to the concentration of Zn2+ and Ag+ when Ecell = 0. (1)
(ii) Why does conductivity of a solution decreases with dilution? (1)
OR
The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9 S cm2mol-1. Calculate the conductivity of this solution. (2)
Can we construct an electrochemical cell with two half-cells composed of ZnSO4 solution and zinc electrodes? Explain your answer.