Advertisements
Advertisements
Question
Can `E_(Cell)^Θ` or `∆_rG^Θ` for cell reaction ever be equal to zero?
Solution
`E_(Cell)^Θ` or `∆_rG^Θ` can never be equal to zero. The only standard electrode potential which is arbitrarily assigned the value zero is the standard hydrogen electrode (SHE). Since everything else is measured concerning SHE; the EΘ cell can never be zero.
APPEARS IN
RELATED QUESTIONS
Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
Calculate the emf of the cell in which the following reaction takes place:
\[\ce{Ni_{(s)} + 2Ag^+ (0.002 M) -> Ni^{2+} (0.160 M) + 2Ag_{(s)}}\]
Given that \[\ce{E^Θ_{cell}}\] = 1.05 V
Relationship between equilibrium constant of the reaction and standard electrode potential of electrochemical cell in which that reaction takes place is ____________.
Under what condition is ECell = 0 or ∆rG = 0?
Unlike dry cell, the mercury cell has a constant cell potential throughout its useful life. Why?
Write the Nernst equation for the cell reaction in the Daniel cell. How will the ECell be affected when concentration of Zn2+ ions is increased?
Assertion: Current stops flowing when ECell = 0.
Reason: Equilibrium of the cell reaction is attained.
Consider figure and answer the question to given below.
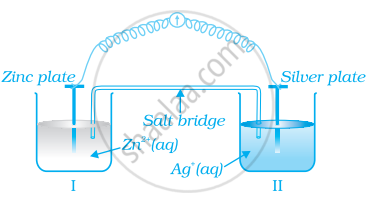
- Redraw the diagram to show the direction of electron flow.
- Is silver plate the anode or cathode?
- What will happen if salt bridge is removed?
- When will the cell stop functioning?
- How will concentration of Zn2+ ions and Ag+ ions be affected when the cell functions?
- How will the concentration of Zn2+ ions and Ag+ ions be affected after the cell becomes ‘dead’?
For the reaction H2 + 12 ⇌ 2Hl, the relation between equilibrium constants Kp and Kc is
For a cell reaction in involving a two electron change, the Stanford e. m. f of the cell is found to be 0.295 V at 25°C. The equilibrium constant of the reaction at 25°C will be
