Advertisements
Advertisements
Question
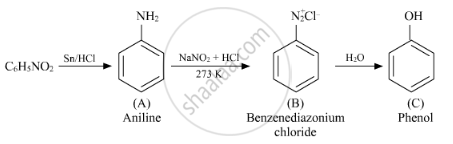
Write the structures of A, B and C in the following reactions :
\[ C_6 H_5 {NO}_2 \to^{Sn/HCI} A \to^{{NaNO}_2 /HCI}_{273 K} B \to^{H_2 O}_∆ C\]
Solution
A: Aniline
B: Benzenediazonium chloride
C: Phenol
APPEARS IN
RELATED QUESTIONS
`CH_3-CH_2-Br"Alcoholic KOH"/""> B " HBR"/"">C"Na/Eather"/"">D`, the Compound D is
(A) ethane
(B) propane
(C) n-butane
(D) n-pentane
Arrange the following compounds in the increasing order of their acid strength:
p-cresol, p-nitrophenol, phenol
Alcohols of low molecular weight are _____________.
Explain why alcohols and ethers of comparable molecular mass have different boiling points?
Assertion: Boiling points of alcohols and ethers are high.
Reason: They can form intermolecular hydrogen-bonding.
A solution of phenol in chloroform when treated with aqueous NaOH gives compound P as a major product. The mass percentage of carbon in P is ______. (to the nearest integer) (Atomic mass: C = 12; H = 1; O = 16)
Arrange the following in order of increasing boiling point:
Ethoxyethane, Butanal, Butanol, n-butane
Assertion (A): Alcohols react both as nucleophiles and electrophiles.
Reason (R): The bond between C–O is broken when alcohols react as nucleophiles.
Select the most appropriate answer from the options given below:
Write a note on Kolbe's reaction
How are the following conversions carried out?
\[\ce{Methyl magnesium bromide -> 2-Methylpropan-2-ol}\]
