Advertisements
Advertisements
Question
`CH_3-CH_2-Br"Alcoholic KOH"/""> B " HBR"/"">C"Na/Eather"/"">D`, the Compound D is
(A) ethane
(B) propane
(C) n-butane
(D) n-pentane
Solution
(C) n-butane
`CH_3-CH_2-Br"Alcoholic KOH"/""> CH_2=CH_2 " HBR"/"">CH_3-CH_2-Br"Na/Eather"/"">Ch_3-CH_2-CH_2-CH_3`,
APPEARS IN
RELATED QUESTIONS
Give reasons for the following: Butan-1-ol has a higher boiling point than diethyl ether.
Give reasons for the following : Phenol is more acidic than ethanol.
Give reasons for the following : Boiling point of ethanol is higher in comparison to methoxymethane.
Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Give simple chemical tests to distinguish between the following pairs of compounds :
Ethanol and phenol
Arrange the following compounds in the increasing order of their acid strength:
p-cresol, p-nitrophenol, phenol
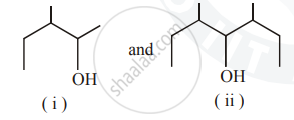
Identify the chiral molecule in the following pair :
Write the structures of A, B and C in the following reactions :
\[ C_6 H_5 {NO}_2 \to^{Sn/HCI} A \to^{{NaNO}_2 /HCI}_{273 K} B \to^{H_2 O}_∆ C\]
Account for the following:
CH3CHO is more reactive than CH3COCH3 towards reaction with HCN.
Write a chemical reaction to show that the open structure of D-glucose contains the following :
Five alcohol groups
Alcohols have high boiling points because of ____________.
Isopropyl alcohol is obtained by reacting which of the following alkenes with concentrated H2SO4 followed by boiling with H2O?
Which statement is not correct about alcohol?
Which one of the following alcohols is least soluble in water?
Arrange the following compounds in increasing order of boiling point. Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
Assertion: Boiling points of alcohols and ethers are high.
Reason: They can form intermolecular hydrogen-bonding.
Arrange the following in the increasing order of their property indicated:
ethanol, ethanoic acid, benzoic acid (boiling point)
A solution of phenol in chloroform when treated with aqueous NaOH gives compound P as a major product. The mass percentage of carbon in P is ______. (to the nearest integer) (Atomic mass: C = 12; H = 1; O = 16)
Assertion (A): Alcohols react both as nucleophiles and electrophiles.
Reason (R): The bond between C–O is broken when alcohols react as nucleophiles.
Select the most appropriate answer from the options given below:
Write a note on Kolbe's reaction
What is esterifications? How is an ester obtained from alcohol or phenol?
How are the following conversions carried out?
Methyl magnesium bromide → 2 -Methylpropan-2-ol.
How are the following conversions carried out?
\[\ce{Methyl magnesium bromide ->2-Methylpropan-2-ol}\]
