Advertisements
Advertisements
Question
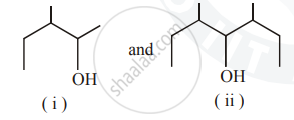
Identify the chiral molecule in the following pair :
Solution
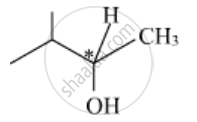
Out of the given molecules, (i) is a chiral molecule since the C-atom (marked as * in the figure) is chiral and different groups are attached to it. This molecule does not have the centre of symmetry.
APPEARS IN
RELATED QUESTIONS
Give reasons for the following : Phenol is more acidic than ethanol.
Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Give simple chemical tests to distinguish between the following pairs of compounds :
Ethanol and phenol
Write the structures of A, B and C in the following reactions :
\[ C_6 H_5 {NO}_2 \to^{Sn/HCI} A \to^{{NaNO}_2 /HCI}_{273 K} B \to^{H_2 O}_∆ C\]
Alcohols have high boiling points because of ____________.
Alcohols of low molecular weight are _____________.
Which statement is not correct about alcohol?
Arrange the following compounds in increasing order of boiling point. Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
Arrange the following in order of increasing boiling point:
Ethoxyethane, Butanal, Butanol, n-butane
Assertion (A): Alcohols react both as nucleophiles and electrophiles.
Reason (R): The bond between C–O is broken when alcohols react as nucleophiles.
Select the most appropriate answer from the options given below:
