Advertisements
Advertisements
Question
How would you account the following :
Transition metals and their compounds show catalytic properties.
Solution
The catalytic activity of transition metals and their compounds can be explained by two basic facts:
1. Owing to their ability to show variable oxidation states and form complexes, transition metals form unstable intermediate compounds. Thus, they provide a new path with lower activation energy, Ea, for a reaction.
2. Transition metals also provide a suitable surface for reactions to occur.
APPEARS IN
RELATED QUESTIONS
Write the major products(s) in the following:
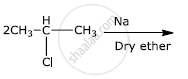
How will you bring about the following conversion?
Benzene to biphenyl
How the following conversion can be carried out?
Benzene to diphenyl
The reaction \[\ce{RX + 2Na + RX -> R - R + 2NaX}\] is called ____________.
Chlorobenzene is prepared commercially by:
Match the reactions given in Column I with the names given in Column II.
| Column I | Column II | |
| (i) | 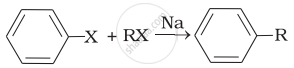 |
(a) Fittig reaction |
| (ii) | 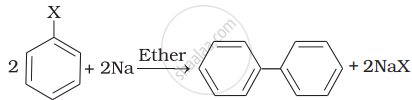 |
(b) Wurtz Fittig reaction |
| (iii) | 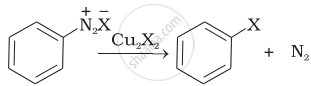 |
(c) Finkelstein reaction |
| (iv) | \[\ce{C2H5Cl + Nal ->[dry acetone] C2H5l + NaCl}\] | (d) Sandmeyer reaction |
Assertion: tert-Butyl bromide undergoes Wurtz reaction to give 2, 2, 3, 3-tetramethylbutane.
Reason: In Wurtz reaction, alkyl halides react with sodium in dry ether to give hydrocarbon containing double the number of carbon atoms present in the halide.
Write balanced equations for a coloured metallic oxide which dissolves in alkalis to yield colourless solutions.
Explain why Grignard reagents should be prepared under anhydrous conditions?
