Advertisements
Advertisements
Question
Account for the following :
Bond angle in NH+4NH4+ is higher than NH3.
Solution
NH3 has a lone pair of electrons that strongly repels the bonded pair of electrons and reduces the bond angle. On the other hand, NH+4NH4+ does not have any lone pair of electrons; hence, there is no lone pair–bond pair repulsion. Thus, the bond angle in NH+4NH4+ is higher than that in NH3.
shaalaa.com
Is there an error in this question or solution?
APPEARS IN
RELATED QUESTIONS
Give reasons for the following:
(i) Bond enthalpy of F2 is lower than that of Cl2.
(ii) PH3 has lower boiling point than NH3.
Identify 'A' and 'B' and rewrite the reactions
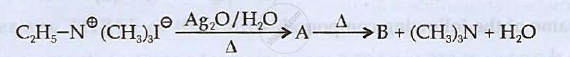
The ligand diethylene triamine is -
(a) monodentate
(b) bidentate
(c) tridentate
(d) tetradentate
Give reasons for the following:
N-N bond is weaker than P-P bond.
CH3 – mg – be is an organometallic compound due to
