Advertisements
Advertisements
Question
Give simple chemical tests to distinguish between the following pairs of compounds: Benzoic acid and Phenol
Solution 1
(ii) Distinguish test between Benzoic acid and Phenol:
NaHCO3 Test: When Benzoic acid reacts with NaHCO3, brisk effervescence of CO2 gas evolved.

Phenol does not give this test.
C6H5OH + NaHCO3 → No Reaction.
Solution 2
Benzoic acid and Phenol : Benzoic acid and phenol can be distinguish by FeCl3 tests. Both reacts with FeCl3 to give different colours. Phenol reacts with FeCl3 to give violet coloured precipitate while benzoic acid give buff coloured precipitate.

Solution 3
When treated with sodium bicarbonate, benzoic acid gives brisk effervescence due to evolution of carbon dioxide gas. No reaction takes place when phenol is treated with NaHCO3.
C6H5COOH + NaHCO3 → C6H5COONa + H2O + CO2
APPEARS IN
RELATED QUESTIONS
Write the final product(s) in each of the following reactions:
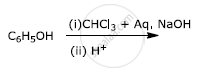
Write the structures of A, B, C, D and E in the following reactions:
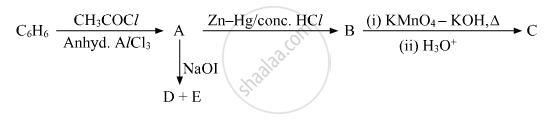
Write the equation involved in the following reaction:
Reimer-Tiemann reaction
Explain the following with an example.
Kolbe’s reaction.
When phenol is treated with excess bromine water, it gives:
On distilling phenol with Zn dust, one gets:
Which of the following species can act as the strongest base?
\[\ce{C2H5OH + SOCl2 ->[Pyridine] C2H5Cl + SO2 + HCl}\]
The above reaction is known as:
Out of o-nitrophenol and p-nitrophenol, which is more volatile? Explain.
Write the chemical equation involved in the following reactions:
Acetylation of salicylic add
