Advertisements
Advertisements
Question
What happens when \[\ce{CH3 - Br}\] is treated with KCN?
Solution
It is a nucleophilic substitution reaction. The nucleophile CN− substitutes Br−.
The reaction is as follows:
\[\ce{CH3BrKCN -> CH3CN + K Br}\]
APPEARS IN
RELATED QUESTIONS
Chlorobenzene is extremely less reactive towards a nucleophilic substitution reaction. Give two reasons for the same.
Write the final product(s) in each of the following reactions:
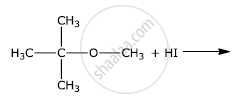
How the following conversion can be carried out?
Chlorobenzene to p-nitrophenol
Give reasons:
The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
Write the product formed on reaction of D-glucose with Br2 water.
What is Grignard reagent?
Write chemical equation in support of your answer.
Out of 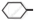 Cl and
Cl and 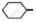 CH2- Cl, which one is more reactive towards nucleophilic substitution reaction and why?
CH2- Cl, which one is more reactive towards nucleophilic substitution reaction and why?
Out of (CH3)3 C-Br and (CH3)3 C-I, which one is more reactive towards SN1 and why?
Arrange the following compounds in increasing order of rate of reaction towards nucleophilic substitution.
| (a) | 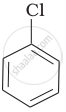 |
| (b) | 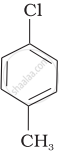 |
| (c) | 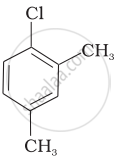 |
Haloarenes are less reactive than haloalkanes and haloalkenes. Explain.
