Advertisements
Advertisements
Question
How will you bring about the following conversion?
Benzoyl chloride to benzaldehyde
Solution
Conversion of Benzoyl chloride to benzaldehyde:
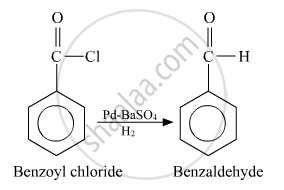
APPEARS IN
RELATED QUESTIONS
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
p-Nitrobenzaldehyde
Write the reaction involved in the Stephen reduction
The oxidation of toluene to benzoic acid can be stopped at the aldehyde stage to give benzaldehyde. The reagent used for the purpose is one of the following.
Match the example given in Column I with the name of the reaction in Column II.
| Column I (Example) |
Column II (Reaction) |
||
| (i) | \[\begin{array}{cc} \phantom{...}\ce{O}\phantom{..............................}\ce{O}\phantom{}\\ \phantom{...}||\phantom{..............................}||\phantom{}\\ \ce{CH3 - C - Cl + H2 ->[Pd - C/BasO4] CH3 - C - H} \end{array}\] |
(a) | Friedel Crafts acylation |
| (ii) | 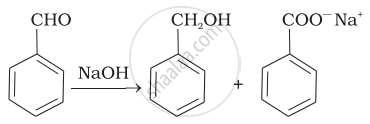 |
(b) | HVZ reaction |
| (iii) | 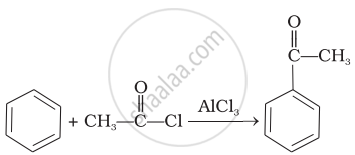 |
(c) | Aldol condensation |
| (iv) | \[\begin{array}{cc} \ce{R - CH2 - COOH ->[Br/Red P] R - CH - COOH}\\ \phantom{.....................}|\\ \phantom{.......................}\ce{Br} \end{array}\] |
(d) | Cannizaro’s reaction |
| (v) | \[\ce{CH3 - CN ->[(i) SnCl2/HCl][(ii) H2O/H+] CH3CHO}\] | (e) | Rosenmund’s reductio |
| (vi) | \[\ce{2CH3CHO ->[NaOH] CH3 - CH = CHCHO}\] | (f) | Stephen’s reaction |
In the chromyl chloride test, the final step results in the formation of a yellow precipitate of the following:
The strongest base among the following
The number of chiral carbon in glucose is:-
The reagent in friedel - craft reaction is:
Reagent used to convert allyl alcohol to acrolein is ______.
An organic compound with molecular formula \[\ce{C7H7NO2}\] exists in three isomeric forms, the isomer ‘A’ has the highest melting point of the three. ‘A’ on reduction gives compound ‘B’ with molecular formula \[\ce{C7H9N}\]. ‘B’ on treatment with \[\ce{NaNO2/HCl}\] at 0-5° C to form compound ‘C’. On treating C with \[\ce{H3PO2}\], it gets converted to D with formula \[\ce{C7H8}\], which on further reaction with \[\ce{CrO2Cl2}\] followed by hydrolysis forms ‘E’ \[\ce{C7H6O}\]. Write the structure of compounds A to E. Write the chemical equations involved.
