Advertisements
Advertisements
Question
An organic compound with molecular formula \[\ce{C7H7NO2}\] exists in three isomeric forms, the isomer ‘A’ has the highest melting point of the three. ‘A’ on reduction gives compound ‘B’ with molecular formula \[\ce{C7H9N}\]. ‘B’ on treatment with \[\ce{NaNO2/HCl}\] at 0-5° C to form compound ‘C’. On treating C with \[\ce{H3PO2}\], it gets converted to D with formula \[\ce{C7H8}\], which on further reaction with \[\ce{CrO2Cl2}\] followed by hydrolysis forms ‘E’ \[\ce{C7H6O}\]. Write the structure of compounds A to E. Write the chemical equations involved.
Solution
Compound A is p-methylnitrobenzene
Compound B is p- methylbenzenamine
Compound C is p-methylbenzenediazoiumchloride
Compound D - Toluene
Compound E - Benzaldehyde
The chemical reactions involved are

APPEARS IN
RELATED QUESTIONS
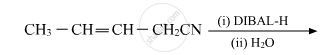
Write the product in the following reaction
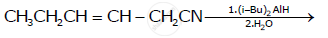
Write the product in the following reaction:
The oxidation of toluene to benzoic acid can be done using which of the following reagents.
An alkene ‘A’ (Mol. formula \[\ce{C5H10}\]) on ozonolysis gives a mixture of two compounds ‘B’ and ‘C’. Compound ‘B’ gives positive Fehling’s test and also forms iodoform on treatment with \[\ce{I2}\] and \[\ce{NaOH}\]. Compound ‘C’ does not give Fehling’s test but forms iodoform. Identify the compounds A, B and C. Write the reaction for ozonolysis and formation of iodoform from B and C.
The general formula CnH2NO2 could be for open chain
The reagent in friedel - craft reaction is:
Explain the following reactions:
Stephan reaction
Predict the reagent for carrying out the following transformations:
Benzoyl chloride to Benzaldehyde
The reaction of benzene with CO and HCl in the presence of anhydrous AlCl3 gives ______.
Write the name of the reaction, structure and IUPAC name of the product formed when:
CH3CH2CN reacts with stannous chloride in the presence of hydrochloric acid, followed by hydrolysis.
