Advertisements
Advertisements
Question
An alkene ‘A’ (Mol. formula \[\ce{C5H10}\]) on ozonolysis gives a mixture of two compounds ‘B’ and ‘C’. Compound ‘B’ gives positive Fehling’s test and also forms iodoform on treatment with \[\ce{I2}\] and \[\ce{NaOH}\]. Compound ‘C’ does not give Fehling’s test but forms iodoform. Identify the compounds A, B and C. Write the reaction for ozonolysis and formation of iodoform from B and C.
Solution
Compound B gives positive Fehling’s test. It shows that it is an aldehyde and gives iodoform test which shows it has \[\ce{^-COCH3}\] group. Compounds C is a ketone because it does not give Fehling’s test but gives iodoform test which shows it also has \[\ce{^-COCH3}\] groups.
Hence compound A is
\[\begin{array}{cc}
\ce{CH3CH = C - CH3}\\
\phantom{....}|\\
\phantom{.......}\ce{CH3}
\end{array}\]
(i)
\[\begin{array}{cc}
\ce{CH3 - CH = C - CH3 ->[(i) O3][(ii) Zn/H2O] \underset{Acetaldehyde}{CH3 - CHO} + O = C - H3}\\
\phantom{........}|\phantom{...................}\ce{\underset{(B)}{(Ethanal)}}\phantom{..........}|\phantom{}\\
\phantom{..........}\ce{\underset{(A)}{\underset{2-Methylbut-2-ene}{CH3}} \phantom{............................}\underset{(C)}{\underset{(Acetone)}{CH3}}}\phantom{...}
\end{array}\]
Other isomer of (A) will not give products corresponding to the given test.
\[\ce{(2NaOH + I2 -> NaOI + NaI + H2O)}\]
(ii)
\[\ce{\underset{(B)}{CH3CHO} + \underset{hypoiodite}{\underset{Sodium}{3NaOI}} -> \underset{accetadehyde}{\underset{Tri-iodo}{CI3CHO}} + 3NaOH}\]
\[\ce{CH3\underset{Iodal}{CHO} + NaOH ->[Hydrolysis] \underset{Iodoform}{CHI3} + HCOONa}\]
(iii)
\[\begin{array}{cc}
\phantom{..}\ce{O}\phantom{............................}\ce{O}\phantom{............}\\
\phantom{.}||\phantom{.............................}||\phantom{...........}\\
\ce{CH3 - \underset{(C)}{C} - CH3 + 3NaOI -> \underset{Tri-iodoacetone}{CI3 - C - CH3} + 3NaOH}
\end{array}\]
\[\begin{array}{cc}
\ce{O}\phantom{.........................................}\\
||\phantom{.........................................}\\
\ce{CH3 - C - CH3 + NaOH ->[Hydrolysis] \underset{Iodoform}{CHl3} + \underset{Sodium acetate}{CH3COONa}}
\end{array}\]
RELATED QUESTIONS
How will you bring about the following conversion?
Benzoyl chloride to benzaldehyde
Ozonolysis of alkenes followed by the reaction with zinc dust and water gives ____________ depending on the substitution pattern of the alkene.
Esters react with DIBAL-H to produce:
Aldehydes are produced on reduction of the following by DIBAL-H:
The oxidation of toluene to benzoic acid can be done using which of the following reagents.
\[\begin{array}{cc}
\ce{CH3 - CH2 - CH - CH3}\\
\phantom{.....}|\\
\phantom{......}\ce{Cl}
\end{array}\] obtained by chlorination of n-butane, will be
When 2 – hydroxyl benzoic acid distilled with zinc dust, it give
Aldehydes are the first oxidation products of ______.
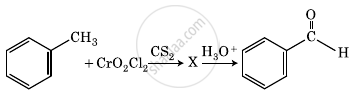
The intermediate compound ‘X’ in the following chemical reaction is:
Reagent used to convert allyl alcohol to acrolein is ______.
