Advertisements
Advertisements
प्रश्न
An alkene ‘A’ (Mol. formula \[\ce{C5H10}\]) on ozonolysis gives a mixture of two compounds ‘B’ and ‘C’. Compound ‘B’ gives positive Fehling’s test and also forms iodoform on treatment with \[\ce{I2}\] and \[\ce{NaOH}\]. Compound ‘C’ does not give Fehling’s test but forms iodoform. Identify the compounds A, B and C. Write the reaction for ozonolysis and formation of iodoform from B and C.
उत्तर
Compound B gives positive Fehling’s test. It shows that it is an aldehyde and gives iodoform test which shows it has \[\ce{^-COCH3}\] group. Compounds C is a ketone because it does not give Fehling’s test but gives iodoform test which shows it also has \[\ce{^-COCH3}\] groups.
Hence compound A is
\[\begin{array}{cc}
\ce{CH3CH = C - CH3}\\
\phantom{....}|\\
\phantom{.......}\ce{CH3}
\end{array}\]
(i)
\[\begin{array}{cc}
\ce{CH3 - CH = C - CH3 ->[(i) O3][(ii) Zn/H2O] \underset{Acetaldehyde}{CH3 - CHO} + O = C - H3}\\
\phantom{........}|\phantom{...................}\ce{\underset{(B)}{(Ethanal)}}\phantom{..........}|\phantom{}\\
\phantom{..........}\ce{\underset{(A)}{\underset{2-Methylbut-2-ene}{CH3}} \phantom{............................}\underset{(C)}{\underset{(Acetone)}{CH3}}}\phantom{...}
\end{array}\]
Other isomer of (A) will not give products corresponding to the given test.
\[\ce{(2NaOH + I2 -> NaOI + NaI + H2O)}\]
(ii)
\[\ce{\underset{(B)}{CH3CHO} + \underset{hypoiodite}{\underset{Sodium}{3NaOI}} -> \underset{accetadehyde}{\underset{Tri-iodo}{CI3CHO}} + 3NaOH}\]
\[\ce{CH3\underset{Iodal}{CHO} + NaOH ->[Hydrolysis] \underset{Iodoform}{CHI3} + HCOONa}\]
(iii)
\[\begin{array}{cc}
\phantom{..}\ce{O}\phantom{............................}\ce{O}\phantom{............}\\
\phantom{.}||\phantom{.............................}||\phantom{...........}\\
\ce{CH3 - \underset{(C)}{C} - CH3 + 3NaOI -> \underset{Tri-iodoacetone}{CI3 - C - CH3} + 3NaOH}
\end{array}\]
\[\begin{array}{cc}
\ce{O}\phantom{.........................................}\\
||\phantom{.........................................}\\
\ce{CH3 - C - CH3 + NaOH ->[Hydrolysis] \underset{Iodoform}{CHl3} + \underset{Sodium acetate}{CH3COONa}}
\end{array}\]
संबंधित प्रश्न
Write the product in the following reaction
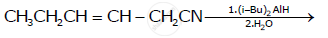
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
p-Nitrobenzaldehyde
Aldehydes are produced on reduction of the following by DIBAL-H:
Name the electrophile produced in the reaction of benzene with benzoyl chloride in the presence of anhydrous \[\ce{AlCl3}\]. Name the reaction also.
Ethylbenzene is generally prepared by acetylation of benzene followed by reduction and not by direct alkylation. Think of a possible reason.
What is the name of the given reaction of preparation of aldehyde?
\[\ce{C3COCl ->[H2][Pd/BaSO4] CH3CHO + HCl}\]
The reaction

Reagent used to convert allyl alcohol to acrolein is ______.
An organic compound with molecular formula \[\ce{C7H7NO2}\] exists in three isomeric forms, the isomer ‘A’ has the highest melting point of the three. ‘A’ on reduction gives compound ‘B’ with molecular formula \[\ce{C7H9N}\]. ‘B’ on treatment with \[\ce{NaNO2/HCl}\] at 0-5° C to form compound ‘C’. On treating C with \[\ce{H3PO2}\], it gets converted to D with formula \[\ce{C7H8}\], which on further reaction with \[\ce{CrO2Cl2}\] followed by hydrolysis forms ‘E’ \[\ce{C7H6O}\]. Write the structure of compounds A to E. Write the chemical equations involved.
Write the name of the reaction, structure and IUPAC name of the product formed when:
CH3CH2CN reacts with stannous chloride in the presence of hydrochloric acid, followed by hydrolysis.
