Advertisements
Advertisements
प्रश्न
Assertion: Aldehydes and ketones, both react with Tollen’s reagent to form silver mirror.
Reason: Both, aldehydes and ketones contain a carbonyl group.
पर्याय
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct statement but reason is wrong statement.
Assertion is wrong statement but reason is correct statement.
Assertion and reason both are correct statements but reasson is not correct explanation of assertion.
उत्तर
Assertion is wrong statement but reason is correct statement.
Explanation:
The silver mirror test can be used to determine Tollen's. \[\ce{[Ag(NH2)2]+ OH-}\]. Only aldehydes, not ketones, react with Tollen's reagent to create silver.
A silver mirror test is not given with this affirmative test.
The carbonyl group is present in both aldehyde and ketone.
APPEARS IN
संबंधित प्रश्न
How is carbolic acid prepared from chlorobenzene?
Write the structures of A and B in the following reactions
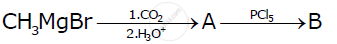
Predict the products of the following reactions:
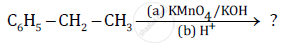
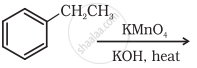
Show how the following compound can be converted to benzoic acid.
Bromobenzene
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
m-Nitrobenzoic acid
Complete the synthesis by giving missing starting material, reagent or product.
How is methoxy benzene prepared from carbolic acid?
The functional group present in triacylglycerol is _______.
Which of the following substance produced acetaldehyde on dry distillation?
Alkaline hydrolysis of C4H8Cl2 gives a compound (A) which on heating with NaOH and I2 produces a yellow precipitate of CHI3. The compound (A) should be ______.
