Advertisements
Advertisements
प्रश्न
An organic compound with molecular formula \[\ce{C7H7NO2}\] exists in three isomeric forms, the isomer ‘A’ has the highest melting point of the three. ‘A’ on reduction gives compound ‘B’ with molecular formula \[\ce{C7H9N}\]. ‘B’ on treatment with \[\ce{NaNO2/HCl}\] at 0-5° C to form compound ‘C’. On treating C with \[\ce{H3PO2}\], it gets converted to D with formula \[\ce{C7H8}\], which on further reaction with \[\ce{CrO2Cl2}\] followed by hydrolysis forms ‘E’ \[\ce{C7H6O}\]. Write the structure of compounds A to E. Write the chemical equations involved.
उत्तर
Compound A is p-methylnitrobenzene
Compound B is p- methylbenzenamine
Compound C is p-methylbenzenediazoiumchloride
Compound D - Toluene
Compound E - Benzaldehyde
The chemical reactions involved are

APPEARS IN
संबंधित प्रश्न
Write the product in the following reaction
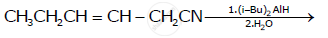
How will you bring about the following conversion?
Benzoyl chloride to benzaldehyde
Predict the product of the following reaction:
Ozonolysis of alkenes followed by the reaction with zinc dust and water gives ____________ depending on the substitution pattern of the alkene.
Can Gatterman-Koch reaction be considered similar to Friedel Craft’s acylation? Discuss.
An aromatic compound ‘A’ (Molecular formula \[\ce{C8H8O}\]) gives positive 2, 4-DNP test. It gives a yellow precipitate of compound ‘B’ on treatment with iodine and sodium hydroxide solution. Compound ‘A’ does not give Tollen’s or Fehling’s test. On drastic oxidation with potassium permanganate it forms a carboxylic acid ‘C’ (Molecular formula \[\ce{C7H6O2}\]), which is also formed along with the yellow compound in the above reaction. Identify A, B and C and write all the reactions involved.
The strongest base among the following
Explain the following reactions:
Stephan reaction
The reaction of benzene with CO and HCl in the presence of anhydrous AlCl3 gives ______.
Reagent used to convert allyl alcohol to acrolein is ______.
