Advertisements
Advertisements
प्रश्न
Name the electrophile produced in the reaction of benzene with benzoyl chloride in the presence of anhydrous \[\ce{AlCl3}\]. Name the reaction also.
उत्तर
\[\begin{array}{cc}
\phantom{}\ce{O}\phantom{.......................}\ce{O}\phantom{...............................}\\
\phantom{}||\phantom{.......................}||\phantom{...............................}\\
\ce{C6HC - Cl + AlCl3 -> C6H5\overset{δ+}{C - Cl} = \overset{δ-}{Alcl3} -> C6H5Co+ + AlCl4}
\end{array}\]
Friedel–Crafts acylation reaction.
APPEARS IN
संबंधित प्रश्न
Esters react with DIBAL-H to produce:
Aldehydes are produced on reduction of the following by DIBAL-H:
Aldehydes are prepared by reducing nitriles to corresponding imines with stannous chloride in the presence of hydrochloric acid. This reaction is called:
Match the example given in Column I with the name of the reaction in Column II.
| Column I (Example) |
Column II (Reaction) |
||
| (i) | \[\begin{array}{cc} \phantom{...}\ce{O}\phantom{..............................}\ce{O}\phantom{}\\ \phantom{...}||\phantom{..............................}||\phantom{}\\ \ce{CH3 - C - Cl + H2 ->[Pd - C/BasO4] CH3 - C - H} \end{array}\] |
(a) | Friedel Crafts acylation |
| (ii) | 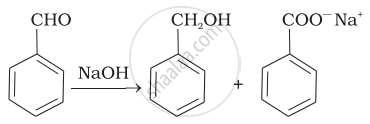 |
(b) | HVZ reaction |
| (iii) | 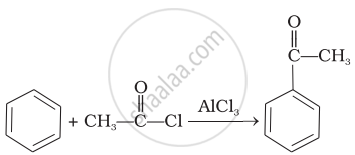 |
(c) | Aldol condensation |
| (iv) | \[\begin{array}{cc} \ce{R - CH2 - COOH ->[Br/Red P] R - CH - COOH}\\ \phantom{.....................}|\\ \phantom{.......................}\ce{Br} \end{array}\] |
(d) | Cannizaro’s reaction |
| (v) | \[\ce{CH3 - CN ->[(i) SnCl2/HCl][(ii) H2O/H+] CH3CHO}\] | (e) | Rosenmund’s reductio |
| (vi) | \[\ce{2CH3CHO ->[NaOH] CH3 - CH = CHCHO}\] | (f) | Stephen’s reaction |
\[\begin{array}{cc}
\ce{CH3 - CH2 - CH - CH3}\\
\phantom{.....}|\\
\phantom{......}\ce{Cl}
\end{array}\] obtained by chlorination of n-butane, will be
Benz aldehyde + NaOH →
The strongest base among the following
The number of chiral carbon in glucose is:-
The general formula CnH2NO2 could be for open chain
The oxidation of toluene to benzaldehyde by chromyl chloride is called ______.
