Advertisements
Advertisements
Question
Account for the following:
N-ethylbenzene sulphonyl amide is soluble in alkali.
Solution
The hydrogen attached to N-Ethylbenzene sulphonamide is acidic in nature. This is due to the presence of a strong electron-withdrawing sulphonyl group. Hence, it is soluble in alkali.
APPEARS IN
RELATED QUESTIONS
Write the product in the following reaction
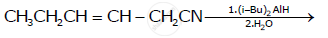
Write the structure of the product of the following reaction:
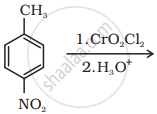
The oxidation of toluene to benzoic acid can be done using which of the following reagents.
Can Gatterman-Koch reaction be considered similar to Friedel Craft’s acylation? Discuss.
An alkene ‘A’ (Mol. formula \[\ce{C5H10}\]) on ozonolysis gives a mixture of two compounds ‘B’ and ‘C’. Compound ‘B’ gives positive Fehling’s test and also forms iodoform on treatment with \[\ce{I2}\] and \[\ce{NaOH}\]. Compound ‘C’ does not give Fehling’s test but forms iodoform. Identify the compounds A, B and C. Write the reaction for ozonolysis and formation of iodoform from B and C.
In the chromyl chloride test, the final step results in the formation of a yellow precipitate of the following:
The strongest base among the following
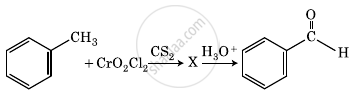
The intermediate compound ‘X’ in the following chemical reaction is:
Convert the following:
Benzoic acid to Benzaldehyde
The reaction of benzene with CO and HCl in the presence of anhydrous AlCl3 gives ______.
