Advertisements
Advertisements
Question
Write the name of the reaction, structure and IUPAC name of the product formed when:
CH3CH2CN reacts with stannous chloride in the presence of hydrochloric acid, followed by hydrolysis.
Solution
Stephen reaction, CH3CH2CHO, Propanal.
APPEARS IN
RELATED QUESTIONS
Predict the product of the following reaction:
Ozonolysis of alkenes followed by the reaction with zinc dust and water gives ____________ depending on the substitution pattern of the alkene.
Aldehydes are prepared by reducing nitriles to corresponding imines with stannous chloride in the presence of hydrochloric acid. This reaction is called:
Match the common names given in Column I with the IUPAC names given in Column II.
| Column I (Common names) |
Column II (IUPAC names) |
||
| (i) | Cinnamaldehyde | (a) | Pentanal |
| (ii) | Acetophenone | (b) | Prop-2-enal |
| (iii) | Valeraldehyde | (c) | 4-Methylpent-3-en-2-one |
| (iv) | Acrolein | (d) | 3-Phenylprop-2-enal |
| (v) | Mesityl oxide | (e) | 1-Phenylethanone |
Match the example given in Column I with the name of the reaction in Column II.
| Column I (Example) |
Column II (Reaction) |
||
| (i) | \[\begin{array}{cc} \phantom{...}\ce{O}\phantom{..............................}\ce{O}\phantom{}\\ \phantom{...}||\phantom{..............................}||\phantom{}\\ \ce{CH3 - C - Cl + H2 ->[Pd - C/BasO4] CH3 - C - H} \end{array}\] |
(a) | Friedel Crafts acylation |
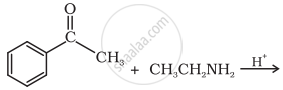
| (ii) | 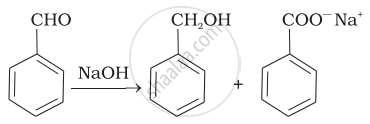 |
(b) | HVZ reaction |
| (iii) | 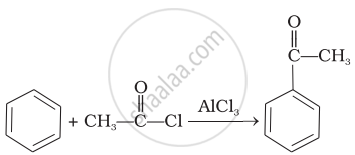 |
(c) | Aldol condensation |
| (iv) | \[\begin{array}{cc} \ce{R - CH2 - COOH ->[Br/Red P] R - CH - COOH}\\ \phantom{.....................}|\\ \phantom{.......................}\ce{Br} \end{array}\] |
(d) | Cannizaro’s reaction |
| (v) | \[\ce{CH3 - CN ->[(i) SnCl2/HCl][(ii) H2O/H+] CH3CHO}\] | (e) | Rosenmund’s reductio |
| (vi) | \[\ce{2CH3CHO ->[NaOH] CH3 - CH = CHCHO}\] | (f) | Stephen’s reaction |
An alkene ‘A’ (Mol. formula \[\ce{C5H10}\]) on ozonolysis gives a mixture of two compounds ‘B’ and ‘C’. Compound ‘B’ gives positive Fehling’s test and also forms iodoform on treatment with \[\ce{I2}\] and \[\ce{NaOH}\]. Compound ‘C’ does not give Fehling’s test but forms iodoform. Identify the compounds A, B and C. Write the reaction for ozonolysis and formation of iodoform from B and C.
The number of chiral carbon in glucose is:-
The reagent in friedel - craft reaction is:
Convert the following:
Benzoic acid to Benzaldehyde
An organic compound with molecular formula \[\ce{C7H7NO2}\] exists in three isomeric forms, the isomer ‘A’ has the highest melting point of the three. ‘A’ on reduction gives compound ‘B’ with molecular formula \[\ce{C7H9N}\]. ‘B’ on treatment with \[\ce{NaNO2/HCl}\] at 0-5° C to form compound ‘C’. On treating C with \[\ce{H3PO2}\], it gets converted to D with formula \[\ce{C7H8}\], which on further reaction with \[\ce{CrO2Cl2}\] followed by hydrolysis forms ‘E’ \[\ce{C7H6O}\]. Write the structure of compounds A to E. Write the chemical equations involved.
