Advertisements
Advertisements
प्रश्न
Draw the geometrical isomers of [Co(en)2Cl2]2+. Which geometrical isomer of [Co(en)2Cl2]2+ is not optically active and why?
उत्तर
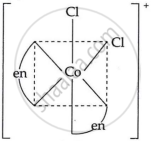 |
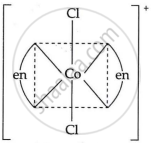 |
| cis-form | trans-form |
The trans isomer of [Co(en)2Cl2]2+ is not optically active because of the plane of symmetry.
APPEARS IN
संबंधित प्रश्न
Explain cationic complexes and anionic complexes of co-ordination compounds.
When a coordination compound CoCl3.6NH3 is mixed with AgNO3, 3moles of AgCl are precipitated per mole of the compound. Write
(i) Structural formula of the complex
Write all the geometrical isomers of [Pt(NH3)(Br)(Cl)(py)] and how many of these will exhibit optical isomers?
What type of isomers are `[Co(NH_3)_5Br]SO_4`and `[Co(NH_3)_5SO_4]Br`?. Give a chemical test to distinguish between them.
Name the type of isomerism exhibited by the following pairs of compound:
(1) (C2H5)2NH and CH3-NH-C3H7
(2) 1 – butanol and 2 methyl-1 -propanol.
Name the type of isomerism that the compound with molecular formula C3H6O2 exhibits. Represent the isomers.
Identify the optically active compounds from the following:
(i) \[\ce{[Co(en)3]^{3+}}\]
(ii) \[\ce{[trans - [Co(en)2Cl2]^+}\]
(iii) \[\ce{cis - [Co(en)2Cl2]^+}\]
(iv) \[\ce{[Cr(NH3)5Cl]}\]
Name the type of isomerism shown by the following pair of compounds:
[Cr(NH3)5Br]SO4 and [Cr(NH3)5SO4]Br
Which of the following molecules has a chiral centre correctly labelled with an asterisk (*)?
Indicate the types of isomerism exhibited by the following complexes and draw the structure for these isomer:
\[\ce{[Pt(NH3)(H2O)Cl2]}\]
