Advertisements
Advertisements
प्रश्न
Identify the optically active compounds from the following:
(i) \[\ce{[Co(en)3]^{3+}}\]
(ii) \[\ce{[trans - [Co(en)2Cl2]^+}\]
(iii) \[\ce{cis - [Co(en)2Cl2]^+}\]
(iv) \[\ce{[Cr(NH3)5Cl]}\]
उत्तर
(i) \[\ce{[Co(en)3]^{3+}}\]
(iii) \[\ce{cis - [Co(en)2Cl2]^+}\]
Explanation:
\[\ce{[Co(en)3]^{3+}}\] and \[\ce{[Co(en)2Cl2]^{2+}}\] are optically active compounds because their mirror images are non-superimposable isomer.
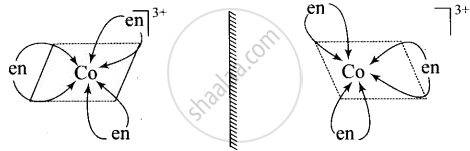
Non – superimposable isomers of \[\ce{[Co(en)3]^{3+}}\]
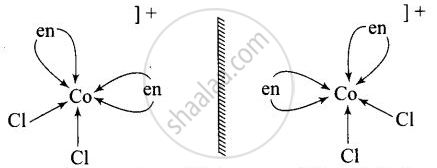
Non – superimosable isomers of \[\ce{[Co(en)2Cl2]^+}\]
APPEARS IN
संबंधित प्रश्न
When a coordination compound CoCl3.6NH3 is mixed with AgNO3, 3moles of AgCl are precipitated per mole of the compound. Write
(i) Structural formula of the complex
Draw one of the geometrical isomers of the complex [Pt (en)2Cl2] +2 which is optically inactive
Draw the geometrical isomers of complex \[\ce{[Pt(en)2Cl2]^2+}\].
Name the type of isomerism shown by the following pair of compounds:
[CoCl(H2O)(NH3)4]Cl2 and [CoCl2(NH3)4]Cl.H2O
Write the IUPAC name of [Co(en)2Cl2]+ ion and draw the structures of its geometrical isomers.
Answer the following question.
Write IUPAC name of the complex [Pt(en)2CI2]. Draw structures of geometrical isomers for this complex.
The IUPAC name for [CoCl(NO2)(en)2]Cl is ____________.
Name the type of isomerism shown by the following pair of compounds:
[Cr(NH3)5Br]SO4 and [Cr(NH3)5SO4]Br
The complex [(Pt(Py)(NH3)BrCl] will have how many geometrical isomers?
Draw the geometrical isomers of [Co(en)2Cl2]2+. Which geometrical isomer of [Co(en)2Cl2]2+ is not optically active and why?
