Advertisements
Advertisements
प्रश्न
Draw the resonance structure of the following compounds;
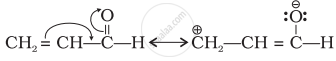
\[\begin{array}{cc}
\ce{CH2 = CH - C = O}\\
\phantom{.........}|\\
\phantom{.........}\ce{H}
\end{array}\]
उत्तर
APPEARS IN
संबंधित प्रश्न
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5OH
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
CH3CH = CHCHO
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5 – CHO
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
\[\ce{C6H5 - \overset{+}{C}H2}\]
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
\[\ce{CH3CH = CH\overset{+}{C}H2}\]
In which of the following representations given below spatial arrangement of group/ atom different from that given in structure ‘A’?
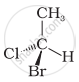 |
| (A) |
| (i) | 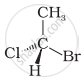 |
| (ii) | 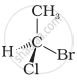 |
| (iii) | 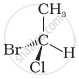 |
| (iv) | 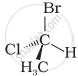 |
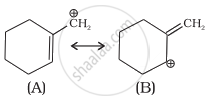
Which of the following ions is more stable? Use resonance to explain your answer.
Draw the resonance structure of the following compounds;
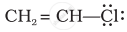
Draw the resonance structure of the following compounds;
CH2 = CH – CH = CH2
Assertion (A): Energy of resonance hybrid is equal to the average of energies of all canonical forms.
Reason (R): Resonance hybrid cannot be presented by a single structure.
