Advertisements
Advertisements
Question
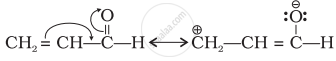
Draw the resonance structure of the following compounds;
\[\begin{array}{cc}
\ce{CH2 = CH - C = O}\\
\phantom{.........}|\\
\phantom{.........}\ce{H}
\end{array}\]
Solution
APPEARS IN
RELATED QUESTIONS
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5OH
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5NO2
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
\[\ce{C6H5 - \overset{+}{C}H2}\]
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
\[\ce{CH3CH = CH\overset{+}{C}H2}\]
The structure of triphenylmethyl cation is given below. This is very stable and some of its salts can be stored for months. Explain the cause of high stability of this cation.

Draw the resonance structure of the following compounds;
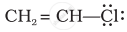
Draw the resonance structure of the following compounds;
CH2 = CH – CH = CH2
Resonance structures of propenal are given below. Which of these resonating structures is more stable? Give reason for your answer.
\[\ce{\underset{I}{CH2 = CH - CH = O} <-> \underset{II}{\overset{⊕}{C}H2 - CH = CH - \overset{Θ}{O}}}\]
Which of the two structures (A) and (B) given below is more stabilised by resonance? Explain.
\[\ce{\underset{(A)}{CH3COOH}}\] and \[\ce{\underset{(B)}{CH3CO\overset{Θ}{O}}}\]
Assertion (A): Energy of resonance hybrid is equal to the average of energies of all canonical forms.
Reason (R): Resonance hybrid cannot be presented by a single structure.
