Advertisements
Advertisements
Question
The structure of triphenylmethyl cation is given below. This is very stable and some of its salts can be stored for months. Explain the cause of high stability of this cation.

Solution
Triphenylmethyl cation is found to be very much stable as this positive charge on methyl carbon is delocalized in three phenyl rings. In each phenyl ring, positive charge is developed on 2 ortho position and para position, i.e., three resonating structures. Total resonating structures given by triphenylmethyl cation are nine. Hence, it is very stable. These structures can be shown as.

APPEARS IN
RELATED QUESTIONS
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
CH3CH = CHCHO
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
\[\ce{C6H5 - \overset{+}{C}H2}\]
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
\[\ce{CH3CH = CH\overset{+}{C}H2}\]
What is the hybridisation of each carbon in H2C = C = CH2.
Show the polarisation of carbon-magnesium bond in the following structure.
CH3 – CH2 – CH2 – CH2 – Mg – X
Which of the following ions is more stable? Use resonance to explain your answer.
Draw the resonance structure of the following compounds;
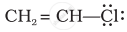
Draw the resonance structure of the following compounds;
\[\begin{array}{cc}
\ce{CH2 = CH - C = O}\\
\phantom{.........}|\\
\phantom{.........}\ce{H}
\end{array}\]
Resonance structures of propenal are given below. Which of these resonating structures is more stable? Give reason for your answer.
\[\ce{\underset{I}{CH2 = CH - CH = O} <-> \underset{II}{\overset{⊕}{C}H2 - CH = CH - \overset{Θ}{O}}}\]
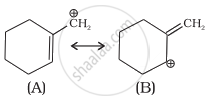
Which of the two structures (A) and (B) given below is more stabilised by resonance? Explain.
\[\ce{\underset{(A)}{CH3COOH}}\] and \[\ce{\underset{(B)}{CH3CO\overset{Θ}{O}}}\]
