Advertisements
Advertisements
Question
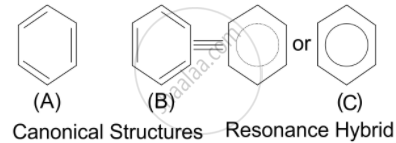
Assertion (A): Energy of resonance hybrid is equal to the average of energies of all canonical forms.
Reason (R): Resonance hybrid cannot be presented by a single structure.
Options
Both A and R are correct and R is the correct explanation of A.
Both A and R are correct but R is not the correct explanation of A.
Both A and R are not correct.
A is not correct but R is correct.
Solution
A is not correct but R is correct.
Explanation:
This is because canonical structures always have more energy than the resonance hybrid. Resonance hybrids are always found to be more stable than any of the canonical structures since the delocalization of electrons lowers the orbitals energy and gives stability.
APPEARS IN
RELATED QUESTIONS
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5OH
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5NO2
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
CH3CH = CHCHO
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
\[\ce{CH3CH = CH\overset{+}{C}H2}\]
In which of the following representations given below spatial arrangement of group/ atom different from that given in structure ‘A’?
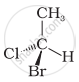 |
| (A) |
| (i) | 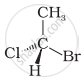 |
| (ii) | 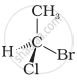 |
| (iii) | 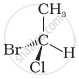 |
| (iv) | 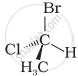 |
Show the polarisation of carbon-magnesium bond in the following structure.
CH3 – CH2 – CH2 – CH2 – Mg – X
The structure of triphenylmethyl cation is given below. This is very stable and some of its salts can be stored for months. Explain the cause of high stability of this cation.

Draw the resonance structure of the following compounds;
CH2 = CH – CH = CH2
Draw the resonance structure of the following compounds;
\[\begin{array}{cc}
\ce{CH2 = CH - C = O}\\
\phantom{.........}|\\
\phantom{.........}\ce{H}
\end{array}\]
Resonance structures of propenal are given below. Which of these resonating structures is more stable? Give reason for your answer.
\[\ce{\underset{I}{CH2 = CH - CH = O} <-> \underset{II}{\overset{⊕}{C}H2 - CH = CH - \overset{Θ}{O}}}\]
