Advertisements
Advertisements
प्रश्न
Energy of an electron in the ground state of the hydrogen atom is –2.18 × 10–18 J. Calculate the ionization enthalpy of atomic hydrogen in terms of J mol–1.
Hint: Apply the idea of mole concept to derive the answer.
उत्तर १
It is given that the energy of an electron in the ground state of the hydrogen atom is –2.18 × 10–18 J.
Therefore, the energy required to remove that electron from the ground state of hydrogen atom is 2.18 × 10–18 J.
∴ Ionization enthalpy of atomic hydrogen = 2.18 × 10–18 J
Hence, ionization enthalpy of atomic hydrogen in terms of J mol–1 = 2.18 × 10–18 × 6.02 × 1023 J mol–1 = 1.31 × 106 J mol–1
उत्तर २
The ionisation enthalpy is for 1 mole atoms. Therefore, ground state energy of the , atoms may be expressed as E (ground state) = ( – 2.18 x 10-18 J) x(6.022 x 1023 mol-1)= -1.312 x 106 J mol-1
Ionisation enthalpy =E∞–E ground state
= 0-(-1.312 x 106mol-1)
= 1.312 x 106 J mol-1.
APPEARS IN
संबंधित प्रश्न
Among the second period elements the actual ionization enthalpies are in the
order Li < B < Be < C < O < N < F < Ne.
Explain why O has lower ΔiH than N and F?
The first ionization enthalpy values (in kJmol–1) of group 13 elements are:-
| B | Al | Ga | In | Tl |
| 801 | 577 | 579 | 558 | 589 |
How would you explain this deviation from the general trend?
Which one of the following statements is incorrect in relation to ionization enthalpy?
Those elements impart colour to the flame on heating in it, the atoms of which require low energy for the ionisation (i.e., absorb energy in the visible region of spectrum). The elements of which of the following groups will impart colour to the flame?
(i) 2
(ii) 13
(iii) 1
(iv) 17
Among the elements \[\ce{B, Al, C}\] and \[\ce{Si}\], which element has the highest first ionisation enthalpy?
Nitrogen has positive electron gain enthalpy whereas oxygen has negative. However, oxygen has lower ionisation enthalpy than nitrogen. Explain.
Arrange the elements \[\ce{N, P, O}\] and \[\ce{S}\] in the order of increasing first ionisation enthalpy. Give reason for the arrangement assigned.
Explain the deviation in ionisation enthalpy of some elements from the general trend by using the given figure.
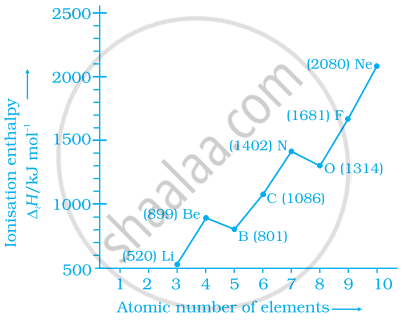
Define ionisation enthalpy. Discuss the factors affecting ionisation enthalpy of the elements and its trends in the periodic table.
Discuss and compare the trend in ionisation enthalpy of the elements of group1 with those of group17 elements.
In general, the property (magnitudes only) that shows an opposite trend in comparison to other properties across a period is ______.
Consider the elements Mg, Al, S, P and Si, the correct increasing order of their first ionization enthalpy is ______.
For the gaseous reaction, \[\ce{K_{(g)} + F_{(g)} -> K^+_{ (g)} + F^-_{ (g)}}\], ΔH was calculated to be 19 kcal/mol under conditions where the cations and anions were prevented by electrostatic separation from combining with each other. The ionisation energy of K is 4.3 eV. The electron affinity of F is ______. (in eV)
`"A"_0/2` atoms of X(g) are converted into X+(g) by absorbing energy E1. `"A"_0/2` ions of X+(g) are converted into X−(g) with release of energy E2. Hence ionization energy and electron affinity of X(g) are ______.
The decreasing order of the second ionization potential of K, Ca and Ba is ______.
Which of the following atoms has the highest first ionization energy?
