Advertisements
Advertisements
प्रश्न
Explain the following term with example.
Covalent bond
उत्तर
Covalent bond
Covalent bond is a chemical bond that involves the sharing of an electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. It is also known as molecular bond. For example: Molecules that have covalent linkages are hydrogen, nitrogen, chlorine, water, and ammonia (H2, N2, Cl2, H2O, NH3).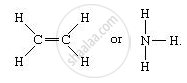
A single line indicates a single bond between two atoms (i.e. involving one electron pair), double lines (=) indicate a double bond between two atoms (i.e. involving two electron pairs), and triple lines (≡) represent a triple bond (C≡O).
shaalaa.com
क्या इस प्रश्न या उत्तर में कोई त्रुटि है?
