Advertisements
Advertisements
प्रश्न
Explain the following observation.
Liquid ammonia bottle is cooled before opening the seal
उत्तर
Liquid ammonia bottle contains the gas under very high pressure. If the bottle is opened as such, then the sudden decrease in pressure will lead to a large increase in the volume of the gas. As a result, the gas will come out of the bottle all of a sudden with force. This will lead to the breakage of the bottle and also causes an accident.
However, if the bottle is cooled under tap water for some time, there will be a decrease in the volume of a gas to a large extent. if the seal is opened now, the gas will come out of the bottle at a slower rate, reduces the chances of an accident.
APPEARS IN
संबंधित प्रश्न
Convert the following temperature from degree Celcius to kelvin.
−15° C
Convert the following temperature from degree Celcius to kelvin.
25° C
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
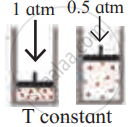 |
______________ |
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
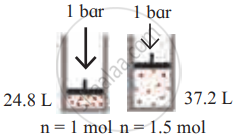 |
______________ |
With the help of the graph answer the following -

At constant temperature, the Graph shows the relationship between pressure and volume. Represent the relation mathematically.
Solve the following.
A syringe has a volume of 10.0 cm3 at pressure 1 atm. If you plug the end so that no gas can escape and push the plunger down, what must be the final volume to change the pressure to 3.5 atm?

The temperatures at which real gases obey the ideal gas laws over a wide range of pressure is called __________.
State Boyle's law.
Name two items that can serve as a model for Gay Lusaac’s law and explain.
A small bubble rises from the bottom of a lake where the temperature and pressure are 6°C and 4 atm. to the water surface, where the temperature is 25°C and pressure is 1 atm. Calculate the final volume in (mL) of the bubble, if its initial volume is 1.5 mL.
