Advertisements
Advertisements
प्रश्न
Explain two neutralisation reactions related to daily life situations.
उत्तर
(a) Indigestion: Due to indigestion a large amount of acid is released into the stomach which causes acidity. This acid is neutralised by taking antacids like milk of magnesia.
(b) Ant’s sting: Ant’s sting releases formic acid in the skin. It can be neutralised by rubbing baking soda or putting in calamine lotion.
APPEARS IN
संबंधित प्रश्न
Explain why Calamine solution is applied on the skin when an ant bites.
Explain why factory waste is neutralised before disposing it into the water bodies.
What is a neutralization reaction?
‘Litmus’, a natural dye is an extract of which of the following?
State whether the following statements are true or false. Correct the false statements.
Common salt dissolved in water turns blue litmus red.
State whether the following statements are true or false. Correct the false statements.
Calamine can be used to treat ant stings.
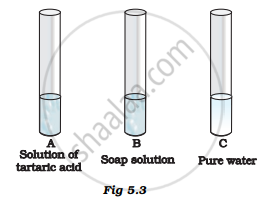
You are provided with three test tubes A, B and C as shown in Figure 5.3 with different liquids. What will you observe when you put
- a piece of blue litmus paper in each test tube.
- a piece of red litmus paper in each test tube.
- a few drops of phenolphthalein solution to each test tube.
Acidity or indigestion in the stomach is due to excessive secretion of ______.
Which chemical is used to neutralize the acidic soil?
How do treat effluents from the industries?
