Advertisements
Advertisements
प्रश्न
From the following elements :
4Be; 9F; 19K; 20Ca
(i) Select the element having one electron is the outermost shell.
(ii) two elements of the same group.
Write the formula of and mention the nature of the compound formed by the union of 19K and element X(2, 8, 7).
उत्तर
(i) Among the given elements, 19K has one electron in the outermost shell. Its electronic configuration is 2, 8, 8, 1.
(ii) The elements 4Be and 20Ca belong to the same group, that is, Group 2. They both have two electrons each in their outermost shells.
Electronic configuration of 4Be is 2, 2.
Electronic configuration of 20Ca is 2, 8, 8, 2.
Atomic number of element X = 17
Electronic configuration of element X is 2, 8, 7.
Number of valence electrons in X = 7
Valency of X = 8 − 7 = 1
Atomic number of K = 19
Electronic configuration of K is 2, 8, 8, 1.
Number of valence electrons in K = 1
Valency of K = 1
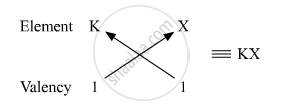
Therefore, the formula of the compound formed when K and element X combine is KX. The compound KX is of ionic nature.
APPEARS IN
संबंधित प्रश्न
Two elements ‘P’ and ‘Q’ belong to the same period of the modern periodic table and are in Group 1 and Group 2, respectively. Compare their following characteristics in tabular form:-
(a) The number of electrons in their atoms
(b) The sizes of their atoms
(c) Their metallic character
(d) Their tendencies to lose electrons
(e) The formula of their oxides
(f) The formula of their chlorides
How does the size of atoms (atomic size) generally vary in going from left to right in a period of the periodic table? Why does it vary this way?
The element which has the maximum number of valence electrons is:
(a) Na
(b) P
(c) Si
(d) A
The atomic numbers of three elements A, B and C are given below:
| Element | Atomic number |
| A | 5 |
| B | 7 |
| C | 10 |
(i) Which element belongs to group 18?
(ii) Which element belongs to group 15?
(iii) Which element belongs to group 13?
(iv) To which period/periods do these elements belong?
An element barium has atomic number 56. Look up its position in the periodic table and answer the following question.
Is it more or less reactive than calcium?
An element 'X' (Atomic number – 20) burns in the presence of oxygen to form a basic oxide.
(a) Identify the element and write its electronic configuration.
(b) State its group number and period number in the Modern Periodic Table.
(c) Write a balanced chemical equation for the reaction when this oxide is dissolved in water.
K, Pb, Ca, Zn (In the increasing order of the reactivity)
How many groups and periods are there in the modern periodic table?
The position of elements A, B, C, D and E in the periodic table are shown below:
|
Group 1 |
Group 2 |
Group 17 |
Group 18 |
|
|
|
|
D |
|
|
B |
C |
|
|
A |
|
|
E |
State which is metals, non-metals, and noble gas in this table.
State the property trends in general of elements on moving from left to right in a period of the periodic table.
