Advertisements
Advertisements
प्रश्न
Give any two uses of infrared waves.
उत्तर
Uses: For therapeutic purposes and long distance photography.
APPEARS IN
संबंधित प्रश्न
Arrange the following radiations in the order of their increasing wavelength:
X-rays, infrared rays, ratio waves, gamma ray and microwaves.
Which radiation is used for satellite communication?
Give one use of ultraviolet radiation.
The wavelength of X-rays is 0.01 Å. Calculate its frequency. State the assumption made, if any.
In a Coolidge tube, electrons strike the target and stop inside it. Does the target get more and more negatively charged as time passes?
Consider a photon of a continuous X-ray coming from a Coolidge tube. Its energy comes from
What happens to the intensity of light from a bulb if the distance from the bulb is doubled? As a laser beam travels across the length of a room, its intensity essentially remains constant. What geometrical characteristic of LASER beam is responsible for the constant intensity which is missing in the case of light from the bulb?
In uranium (Z = 92) the K absorption edge is 0.107 Å and the Kα line is 0.126 Å, and the wavelength of the L absorption edge is ______.
Below is an incomplete table showing the arrangement of electromagnetic spectrum in the increasing order of their wavelength. Complete the table:
| Gamma ray | X - ray | UV rays | Visible rays | Infrared | A | Radio waves |
- Identify the radiation A.
- Name the radiation used to detect fracture in bones.
- Name one property common to both A and Radio waves.
In an atom X, electrons absorb the energy from an external source. This energy “excites” the electrons from a lower-energy level to a higher-energy level around the nucleus of the atom. When electrons return to the ground state, they emit photons.
The figure below is the energy level diagram of atom X with three energy levels, E1 = 0.00eV, E2 = 1.78eV and E3 = 2.95eV. The ground state is considered 0 eV for reference. The transition of electrons takes place between levels E1 and E2.
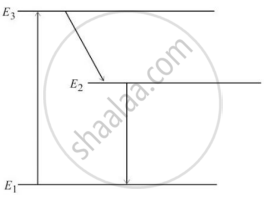
- What wavelength of radiation is needed to excite the atom to energy level E2 from E1?
- Suppose the external source has a power of 100 W. What would be the rate of photon emission?
