Advertisements
Advertisements
प्रश्न
Give a balanced equation when dilute hydrochloric acid is added to : Calcium carbonate
उत्तर
CaCO3 + 2HCI → CaCl2 + H2O +CO2
APPEARS IN
संबंधित प्रश्न
Name a chloride which is solube in excess of ammonium hydroxide
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl -> \underline{\phantom{..........}}}\].
Name the gas evolved when dilute hydrochloric acid is added to: Calcium carbonate
Give a balanced equation when dilute hydrochloric acid is added to : Potassium bisulphite
How will you prove that hydrochloric acid contains
- hydrogen
- chlorine?
Write equations for the reactions.
Convert Hydrochloric acid to nascent chlorine.
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.

Complete and balance the following reaction, state whether dilute or cone. acid is used.
\[\ce{NH4OH + HCl->}\]
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.
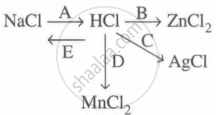
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.

