Advertisements
Advertisements
प्रश्न
Give the formula of the compound that would be formed by the combination of the following pair of elements:
K and H
उत्तर
KH
APPEARS IN
संबंधित प्रश्न
Fill in the blank in the following sentence:
The number of single covalent bonds in C2H2 molecule are ...........
What type of bonding would you expect between Hydrogen and Chlorine?
Draw the electron-dot structure of N2 and state the type of bonding.
State one use of diamond which depends on its 'extraordinary brilliance' and one use of graphite which depends on its being 'black and quite soft'.
Two non-metals combine with each other by the sharing of electrons to form a compound X.
(a) What type of chemical bond is present in X?
(b) State whether X will have a high melting point or low melting point.
(c) Will it be a good conductor of electricity or not?
(d) Will it dissolve in an organic solvent or not?
An element A has 4 valence electrons in its atom whereas element B has only one valence electron in its atom. The compound formed by A and B does not conduct electricity. What is the nature of chemical bond in the compound formed? Give its electron-dot structure.
Complete the following:
In case of non-polar covalent bond, the covalent bond is formed in the ______ of atoms and shared electrons are ______ distributed. (corner, middle, equally, unequally)
Match the pairs.
| Group 'A' | Group 'B' |
| a. C2H6 | 1. Unsaturated hydrocarbon |
| b. C2H2 | 2. Molecular formula of an alcohol |
| c. CH4O | 3. Saturated hydrocarbon |
| d. C3H6 | 4. Triple bond |
Explain the following term with example.
Alkane
Write answer as directed.
What causes the existance of very large number of carbon compound ?
The molecule which contains a triple covalent bond is ______.
State the type of bond formed, and draw Lewis structure of water.
Potassium chloride is an electrovalent compound, while hydrogen chloride is a covalent compound, But, both conduct electricity in their aqueous solutions. Explain.
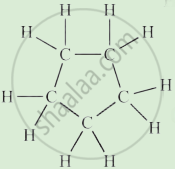
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
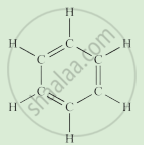 |
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
 |
Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of
The table shows the electronic structures of four elements.
| Element | Electronic Structure |
| P | 2, 6 |
| Q | 2, 8, 1 |
| R | 2, 8, 7 |
| S | 2, 8, 8 |
- Identify which element(s) will form covalent bonds with carbon.
- “Carbon reacts with an element in the above table to form several compounds.” Give suitable reason.
In covalent compounds, the bond is formed due to ______ of electrons.
Explain dipole (polar) molecule by taking hydrogen chloride as an example.
