Advertisements
Advertisements
प्रश्न
\[\ce{H2O2 -> H2O + O2}\]
This represents ______.
विकल्प
Oxidation of H2O2
Reduction of H2O2
Disproportionation of H2O2
Acidic nature of H2O2
MCQ
रिक्त स्थान भरें
उत्तर
\[\ce{H2O2 -> H2O + O2}\]
This represents Disproportionation of H2O2.
Explanation:
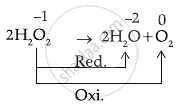
It is known as a disproportionation reaction because the same elements are oxidised and reduced.
shaalaa.com
Oxidation Number - Types of Redox Reactions
क्या इस प्रश्न या उत्तर में कोई त्रुटि है?
