Advertisements
Advertisements
प्रश्न
Heterolytic fission of C–C bond results in the formation of ______.
विकल्प
free radical
Carbanion
Carbocation
Carbanion and Carbocation
उत्तर
Heterolytic fission of C–C bond results in the formation of carbanion and carbocation.
APPEARS IN
संबंधित प्रश्न
For the following reactions
- \[\ce{CH3CH2CH2Br + KOH → CH3 – CH = CH2 + KBr + H2O}\]
- \[\ce{(CH3)3CBr + KOH → (CH3)3COH + KBr}\]
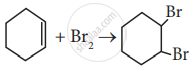
Which of the following statement is correct?
What is the hybridisation state of benzyl carbonium ion?
Which of the following species is not electrophilic in nature?
Which of the group has highest + I effect?
- I effect is shown by ______.
Which of the following carbocation will be most stable?
Which of the following represent a set of nucleophiles?
Write a short note on resonance.
Show the heterolysis of a covalent bond by using curved arrow notation and complete the following equation. Identify the nucelophile.
\[\ce{CH3 – O – CH3 + HI →}\]
Explain inductive effect with suitable example.
