Advertisements
Advertisements
प्रश्न
Identify A to E and also explain the reactions involved.
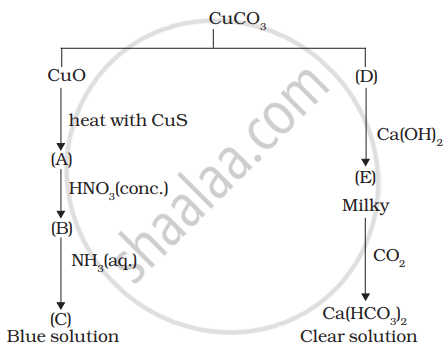
उत्तर
A =
B =
C =
D =
E =
F =
G =
APPEARS IN
संबंधित प्रश्न
The elements of 3d transition series are given as: Sc Ti V Cr Mn Fe Co
Answer the following: Which element has the highest m.p?
How would you account for the following?
Zr (Z = 40) and Hf (Z = 72) have almost identical radii.
Two metallic elements A and B have the following standard oxidation potentials: A = 0·40v B = - 0·80v. What would you expect if element A was added to an aqueous salt solution of element B? Give a reason for your answer.
The second and third rows of transition elements resemble each other much more than they resemble the first row. Explain why?
The halides of transition elements become more covalent with increasing oxidation state of the metal. Why?
Answer the following question:
Which element of the first transition series has highest third ionisation enthalpy?
The element with atomic number 53 belongs to
Catalytic hydrogenation of benzene gives
On strong heating AgNO3, the gases evolved are:-
Agcl is soluble in NH4OH. The solubility is due to the information of:-
