Advertisements
Advertisements
प्रश्न

- Identify the gasses evolved at the anode and cathode in the above experimental set up.
- Name the process that occurs. Why is it called so?
- Illustrate the reaction of the process with the help of a chemical equation.
उत्तर
- Anode: Chlorine; Cathode: Hydrogen
- Chlor alkali process as the products obtained are alkali, chlorine gas and hydrogen gas.
- \[\ce{2NaCl(aq) + 2H2O(I) ->[Electric Current] 2NaOH(aq) + Cl2 (g) + H2(g)}\]
APPEARS IN
संबंधित प्रश्न
Decomposition reactions require energy either in the form of heat or light or electricity for breaking down the reactants. Write one equation each for decomposition reactions where energy is supplied in the form of heat, light and electricity
Rewrite the following statements by selecting the correct options:
\[\ce{CaCO3 ->[\Delta] CaO + CO2 ^}\] is a __________ reaction.
What type of chemical reaction take place when lime-stone is heated?
Identify the type of following reaction :
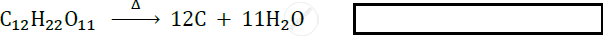
A student wants to study a decomposition reaction by taking ferrous sulphate crystals. Write two precautions he must observe while performing the experiment.
Answer the following question.
2 g of silver chloride is taken in a china dish and the china dish is placed in sunlight for some time. What will be your observation in this case? Write the chemical reaction involved in the form of a balanced chemical equation. Identify the type of chemical reaction.
Differentiate between direct combination reaction and a decomposition reaction.
Which one of the following processes involves chemical reactions?
Complete the following reaction:
\[\ce{C_12H_22O11->[Heat]}\] ______ + ______.
What is observed when silver chloride is exposed to sunlight? Give the type of reaction involved.
