Advertisements
Advertisements
Question

- Identify the gasses evolved at the anode and cathode in the above experimental set up.
- Name the process that occurs. Why is it called so?
- Illustrate the reaction of the process with the help of a chemical equation.
Solution
- Anode: Chlorine; Cathode: Hydrogen
- Chlor alkali process as the products obtained are alkali, chlorine gas and hydrogen gas.
- \[\ce{2NaCl(aq) + 2H2O(I) ->[Electric Current] 2NaOH(aq) + Cl2 (g) + H2(g)}\]
APPEARS IN
RELATED QUESTIONS
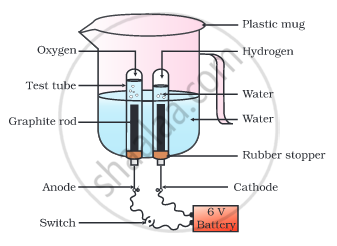
Why is the amount of gas collected in one of the test tubes in the following Activity double of the amount collected in the other? Name this gas.
- Take a plastic mug. Drill two holes at its base and fit rubber stoppers in these holes. Insert carbon electrodes in these rubber stoppers as shown in the following Fig.
- Connect these electrodes to a 6 volt battery.
- Fill the mug with water such that the electrodes are immersed. Add a few drops of dilute sulphuric acid to the water.
- Take two test tubes filled with water and invert them over the two carbon electrodes.
- Switch on the current and leave the apparatus undisturbed for some time.
- You will observe the formation of bubbles at both the electrodes. These bubbles displace water in the test tubes.
- Is the volume of the gas collected the same in both the test tubes?
• Once the test tubes are filled with the respective gases, remove them carefully. - Test these gases one by one by bringing a burning candle close to the mouth of the test tubes.
Caution: This step must be performed carefully by the teacher.
- What happens in each case?
- Which gas is present in each test tube?
A solid substance P which is very hard is used in the construction of many buildings, especially flooring. When substance P is heated strongly, it decomposes to form another solid Q and a gas R is given out. Solid Q reacts with water with the release of a lot of heat to form a substance S. When gas R is passed into a clear solution of substance S, then a white precipitate of substance T is formed. The substance T has the same chemical composition as starting substance P.
(a) What is substance P? Write its common name as well as chemical formula.
(b) What is substance Q?
(c) What is gas R?
(d) What is substance S? What is its clear solution known as?
(e) What is substance T? Name any two natural forms in which substance T occurs in nature.
Give one example of a decomposition reaction which is carried out by applying heat.
What type of chemical reaction take place when electricity is passed through water?
Two metals X and Y form the salts XSO4 and Y2SO4, respectively. The solution of salt XSO4 is blue in colour whereas that of Y2SO4 is colourless. When barium chloride solution is added to XSO4 solution, then a white precipitate Z is formed alongwith a salt which turns the solution green. And when barium chloride solution is added to Y2SO4 solution, then the same white precipitate Z is formed alongwith colourless common salt solution.
(a) What could the metals X and Y be?
(b) Write the name and formula of salt XSO4.
(c) Write the name and formula of salt Y2SO4.
(d) What is the name and formula of white precipitate Z?
(e) Write the name and formula of the salt which turns the solution green in the first case.
MULTIPLE CHOICE QUESTIONS
Tick the most appropriate answer.
Thermal decomposition of a substance is brought about with the help of
- reactants
- water
- wind
- heat
Give scientific reason.
When the gas formed on heating limestone, is passed through freshly prepared lime water, the lime water turns milky.
(a) Design an activity to demonstrate the decomposition reaction of lead nitrate.
(b) Draw labelled diagram of the experimental set-up. List two main observations.
(c) Write balanced chemical equation for the reaction stating the physical state of the reactant and the products.
Classify the following reaction into different type:
\[\ce{2KClO3(s)->[\Delta] 2KCl(aq) + 3O2(g)}\]
Marble statues are corroded or stained rain water. Identify the main reason.