English Medium
Academic Year: 2022-2023
Date: March 2023
Duration: 3h
Advertisements
General Instructions:
- This question paper consists of 39 questions in 5 sections.
- All questions are compulsory. However, an internal choice is provided in some questions. A student is expected to attempt only one of these questions.
- Section A consists of 20 objective type questions carrying 1 mark each.
- Section B consists of 6 Very Short questions carrying 02 marks each. Answers to these questions should be in the range of 30 to 50 words.
- Section C consists of 7 Short Answer type questions carrying 03 marks each. Answers to these questions should be in the range of 50 to 80 words
- Section D consists of 3 Long Answer type questions carrying 05 marks each. Answers to these questions should be in the range of 80 to 120 words.
- Section E consists of 3 source-based/case-based units of assessment of 04 marks each with sub-parts.
The change in colour of the moist litmus paper in the given set up is due to
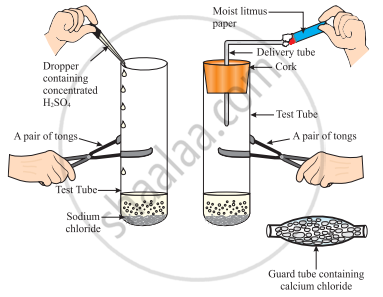
- presence of acid
- presence of base
- presence of H⁺(aq) in the solution
- presence of Litmus which acts as an indicator
i and ii
Only ii
Only iii
Only iv
Chapter: [0.02] Acids, Bases and Salts
In the redox reaction:
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
MnO2 is reduced to MnCl2 & HCl is oxidized to H2O
MnO2 is reduced to MnCl2 & HCl is oxidized to Cl2
MnO2 is oxidized to MnCl2 & HCl is reduced to Cl2
MnO2 is oxidized to MnCl2 & HCl is reduced to H2O
Chapter: [0.01] Chemical Reactions and Equations
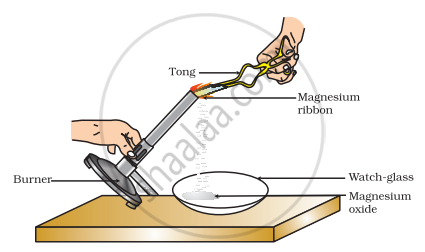
Which of the following is the correct observation of the reaction shown in the above set up?
Brown powder of Magnesium oxide is formed.
Colourless gas which turns lime water milky is evolved.
Magnesium ribbon burns with brilliant white light.
Reddish brown gas with a smell of burning Sulphur has evolved.
Chapter: [0.01] Chemical Reactions and Equations
With the reference to four gases CO2, CO, Cl2 and O2, which one of the options in the table is correct?
| Acidic oxide | Used in treatment of water |
Product of respiration |
Product of incomplete combustion |
| CO | Cl2 | O2 | CO |
| Acidic oxide | Used in treatment of water |
Product of respiration |
Product of incomplete combustion |
| CO2 | Cl2 | CO2 | CO |
| Acidic oxide | Used in treatment of water |
Product of respiration |
Product of incomplete combustion |
| CO2 | O2 | O2 | CO2 |
| Acidic oxide | Used in treatment of water |
Product of respiration |
Product of incomplete combustion |
| CO | O2 | CO2 | CO2 |
Chapter: [0.05] Life Processes [0.05] Life Processes
On placing a copper coin in a test tube containing green ferrous sulphate solution, it will be observed that the ferrous sulphate solution ______.
turns blue, and a grey substance is deposited on the copper coin.
turns colourless and a grey substance is deposited on the copper coin
turns colourless and a reddish–brown substance is deposited on the copper coin.
remains green with no change in the copper coin.
Chapter: [0.02] Acids, Bases and Salts
Anita added a drop each of diluted acetic acid and diluted hydrochloric acid on pH paper and compared the colors. Which of the following is the correct conclusion?
pH of acetic acid is more than that of hydrochloric acid.
pH of acetic acid is less than that of hydrochloric acid.
Acetic acid dissociates completely in aqueous solution.
Acetic acid is a strong acid.
Chapter: [0.04] Carbon and its Compounds
The formulae of four organic compounds are shown below. Choose the correct option
| A | B | C | D |
| \[\begin{array}{cc} \phantom{.}\ce{H}\phantom{......}\ce{H}\\ \phantom{.}\backslash\phantom{.....}/\\ \ce{C = C}\\ /\phantom{.....}\backslash\\ \ce{H}\phantom{......}\ce{H} \end{array}\] |
\[\begin{array}{cc} \phantom{........}\ce{H}\phantom{.....}\ce{O}\\ \phantom{.......}|\phantom{....}//\\ \ce{H - C - C}\\ \phantom{......}|\phantom{.....}\backslash\\ \phantom{...........}\ce{H}\phantom{.....}\ce{O - H} \end{array}\] |
\[\begin{array}{cc}
|
\[\begin{array}{cc} \ce{H}\phantom{...}\ce{H}\phantom{....}\\ |\phantom{....}|\phantom{....}\\ \ce{H - C - C - O - H}\\ |\phantom{....}|\phantom{.....}\\ \ce{H}\phantom{....}\ce{H}\phantom{.....} \end{array}\] |
A and B are unsaturated hydrocarbons
C and D are saturated hydrocarbons
Addition of hydrogen in presence of catalyst changes A to C
Addition of potassium permanganate changes B to D
Chapter: [0.04] Carbon and its Compounds
In the given transverse section of the leaf identify the layer of cells where maximum photosynthesis occurs.
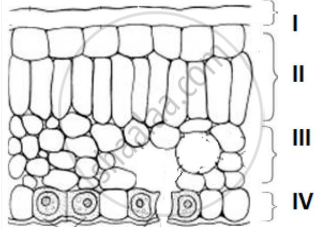
I, II
II, III
III, IV
I, IV
Chapter: [0.05] Life Processes
Observe the experimental setup shown below. Name the chemical indicated as ‘X’ that can absorb the gas which is evolved as a byproduct of respiration.

NaOH
KOH
Ca (OH)2
K2CO3
Chapter: [0.05] Life Processes
If a tall pea plant is crossed with a pure dwarf pea plant then, what percentage of F1 and F2 generation respectively will be tall?
25%, 25%
50%, 50%
75%,100%
100%, 75%
Chapter: [0.08] Heredity
Observe the three figures given below. Which of the following depicts tropic movements appropriately?
 |
 |
 |
| A | B | C |
B and C
A and C
B only
C only
Chapter: [0.06] Control and Co-ordination
The diagram shown below depicts pollination. Choose the options that will show a maximum variation in the offspring.
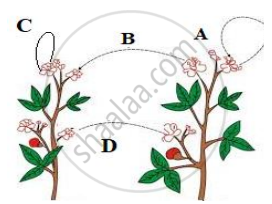
A, B and C
B and D
B, C and D
A and C
Chapter: [0.07] How do Organisms Reproduce? [0.08] Heredity
A complete circuit is left on for several minutes, causing the connecting copper wire to become hot. As the temperature of the wire increases, the electrical resistance of the wire
decreases
remains the same
increases
increases for some time and then decreases
Chapter: [0.11] Electricity
A copper wire is held between the poles of a magnet
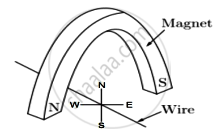
The current in the wire can be reversed. The pole of the magnet can also be changed over. In how many of the four directions shown can the force act on the wire?
1
2
3
4
Chapter: [0.12] Magnetic Effects of Electric Current
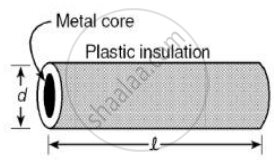
Plastic insulation surrounds a wire having diameter d and length l as shown above. A decrease in the resistance of the wire would be produced by an increase in the ______.
length l of the wire
diameter d of the wire
temperature of the wire
thickness of the plastic insulation
Chapter: [0.11] Electricity
Which of the following pattern correctly describes the magnetic field around a long straight wire carrying current?
straight lines perpendicular to the wire.
straight lines parallel to the wire.
radial lines originating from the wire.
concentric circles centred around the wire.
Chapter: [0.12] Magnetic Effects of Electric Current
These consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion: Silver bromide decomposition is used in black and white photography.
Reason: Light provides energy for this exothermic reaction.
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
Assertion is true but Reason is false
Assertion is False but Reason is true
Chapter: [0.01] Chemical Reactions and Equations
Assertion: Height in pea plants is controlled by the efficiency of enzymes and is thus genetically controlled.
Reason: Cellular DNA is the information source for making proteins in the cell.
Both Assertion and Reason are true and Reason is the correct explanation of Assertion.
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion.
Assertion is true but Reason is false.
Assertion is False but Reason is true.
Chapter: [0.08] Heredity
These consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion: Amphibians can tolerate mixing of oxygenated and deoxygenated blood.
Reason: Amphibians are animals with two chambered heart
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
Assertion is true but Reason is false
Assertion is False but Reason is true
Chapter: [0.05] Life Processes
These consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion: On freely suspending a current – carrying solenoid, it comes to rest in Geographical N-S direction.
Reason: One end of current carrying straight solenoid behaves as a North pole and the other end as a South pole, just like a bar magnet.
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion
Assertion is true but Reason is false
Assertion is False but Reason is true
Chapter: [0.12] Magnetic Effects of Electric Current
Advertisements
A clear solution of slaked lime is made by dissolving Ca(OH)2 in an excess of water. This solution is left exposed to air. The solution slowly goes milky as a faint white precipitate forms. Explain why a faint white precipitate forms, support your response with the help of a chemical equation.
Chapter: [0.01] Chemical Reactions and Equations
Keerti added dilute Hydrochloric acid to four metals and recorded her observations as shown in the table given below:
| Metal | Gas Evolved |
| Copper | Yes |
| Iron | Yes |
| Magnesium | No |
| Zinc | Yes |
Select the correct observation(s) and give chemical equation(s) of the reaction involved.
Chapter: [0.03] Metals and Non Metals
How is the mode of action in beating of the heart different from reflex actions? Give four examples.
Chapter: [0.06] Control and Co-ordination
Patients whose gallbladder are removed are recommended to eat less oily food. Why?
Chapter: [0.05] Life Processes
Name the substances other than water, that are reabsorbed during urine formation. What are the two parameters that decide the amount of water that is reabsorbed in the kidney?
Chapter: [0.05] Life Processes
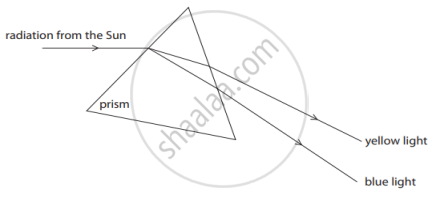
State the phenomena observed in the above diagram. Explain with reference to the diagram, which of the two lights mentioned above will have the higher wavelength?
Chapter:
How will you use two identical prisms so that a narrow beam of white light incident on one prism emerges out of the second prism as white light? Draw the diagram.
Chapter: [0.1] The Human Eye and the Colourful World
A lot of waste is generated in neighborhood. However, almost all of it is biodegradable. What impact will it have on the environment or human health?
Chapter: [0.13] Our Environment

Identify the types of reaction mentioned above. Give one example for type in the form of a balanced chemical equation.
Chapter: [0.01] Chemical Reactions and Equations

Identify the types of reaction mentioned above. Give one example for type in the form of a balanced chemical equation.
Chapter: [0.01] Chemical Reactions and Equations

- Identify the gasses evolved at the anode and cathode in the above experimental set up.
- Name the process that occurs. Why is it called so?
- Illustrate the reaction of the process with the help of a chemical equation.
Chapter: [0.01] Chemical Reactions and Equations
The leaves of a plant were covered with aluminium foil, how would it affect the physiology of the plant?
Chapter: [0.13] Our Environment
How is lymph an important fluid involved in transportation? If lymphatic vessels get blocked, how would it affect the human body? Elaborate.
Chapter: [0.05] Life Processes
Rohit wants to have an erect image of an object using a converging mirror of focal length 40 cm.
- Specify the range of distance where the object can be placed in front of the mirror. Justify.
- Draw a ray diagram to show image formation in this case.
- State one use of the mirror based on the above kind of image formation.
Chapter: [0.09] Light - Reflection and Refraction
A lens of focal length 5 cm is being used by Debashree in the laboratory as a magnifying glass. Her least distance of distinct vision is 25 cm.
- What is the magnification obtained by using the glass?
- She keeps a book at a distance 10 cm from her eyes and tries to read. She is unable to read. What is the reason for this?
Chapter: [0.09] Light - Reflection and Refraction
Ravi kept a book at a distance of 10 cm from the eyes of his friend Hari. Hari is not able to read anything written in the book. Give reasons for this?
Chapter: [0.09] Light - Reflection and Refraction
Advertisements
A student fixes a white sheet of paper on a drawing board. He places a bar magnet in the centre and sprinkles some iron filings uniformly around the bar magnet. Then he taps gently and observes that iron filings arrange themselves in a certain pattern.
- Why do iron filings arrange themselves in a particular pattern?
- Which physical quantity is indicated by the pattern of field lines around the bar magnet?
- State any two properties of magnetic field lines.
Chapter: [0.12] Magnetic Effects of Electric Current
A compass needle is placed near a current carrying wire. State your observations for the following cases and give reasons for the same in each case -
- Magnitude of electric current in wire is increased.
- The compass needle is displaced away from the wire.
Chapter: [0.12] Magnetic Effects of Electric Current
Why is damage to the ozone layer a cause for concern? What steps are being taken to limit this damage?
Chapter: [0.13] Our Environment
Shristi heated Ethanol with a compound A in presence of a few drops of concentrated sulphuric acid and observed a sweet smelling compound B is formed. When B is treated with sodium hydroxide it gives back Ethanol and a compound C.
- Identify A and C
- Give one use each of compounds A and B.
- Write the chemical reactions involved and name the reactions.
Chapter: [0.04] Carbon and its Compounds
What is the role of concentrated Sulphuric acid when it is heated with Ethanol at 443 K. Give the reaction involved.
Chapter: [0.04] Carbon and its Compounds
Reshu by mistake forgot to label the two test tubes containing Ethanol and Ethanoic acid. Suggest an experiment to identify the substances correctly? Illustrate the reactions with the help of chemical equations.
Chapter: [0.04] Carbon and its Compounds
Why is it not possible to reconstruct the whole organism from a fragment in complex multicellular organisms?
Chapter: [0.07] How do Organisms Reproduce?
Sexual maturation of reproductive tissues and organs are necessary link for reproduction. Elucidate.
Chapter: [0.07] How do Organisms Reproduce?
How are variations useful for species if there is drastic alteration in the niches?
Chapter: [0.07] How do Organisms Reproduce? [0.08] Heredity
Explain how the uterus and placenta provide necessary conditions for proper growth and development of the embryo after implantation?
Chapter: [0.07] How do Organisms Reproduce?

The diagram above is a schematic diagram of a household circuit. The house shown in the above diagram has 5 usable spaces where electrical connections are made. For this house, the mains have a voltage of 220 V and the net current coming from the mains is 22A.
- What is the mode of connection to all the spaces in the house from the mains?
- The spaces 5 and 4 have the same resistance and spaces 3 and 2 have respective resistances of 20Ω and 30Ω. Space 1 has a resistance double that of space 5. What is the net resistance for space 5.
- What is the current in space 3?
- What should be placed between the main connection and the rest of the house’s electrical appliances to save them from accidental high electric current?
Chapter: [0.11] Electricity
Two students decided to investigate the effect of water and air on iron object under identical experimental conditions. They measured the mass of each object before placing it partially immersed in 10 ml of water. After a few days, the object were removed, dried and their masses were measured. The table shows their results.
| Student | Object | Mass of Object before Rusting in g |
Mass of the coated object in g |
| A | Nail | 3.0 | 3.15 |
| B | Thin plate | 6.0 | 6.33 |
What might be the reason for the varied observations of the two students?
Chapter: [0.11] Electricity
In a set up the students coated iron nails with zinc metal and noted that, iron nails coated with zinc prevents rusting. They also observed that zinc initially acts as a physical barrier, but an extra advantage of using zinc is that it continues to prevent rusting even if the layer of zinc is damaged. Name this process of rust prevention and give any two other methods to prevent rusting.
Chapter: [0.11] Electricity
In which of the following applications of Iron, rusting will occur most? Support your answer with valid reason.
| A | B | C | D |
 |
 |
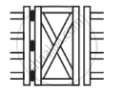 |
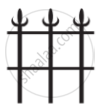 |
A - Iron Bucket electroplated with Zinc
B - Electricity cables having iron wires covered with aluminium
C - Iron hinges on a gate
D - Painted iron fence
Chapter: [0.11] Electricity
Pooja has green eyes while her parents and brother have black eyes. Pooja’s husband Ravi has black eyes while his mother has green eyes and father has black eyes.
a. On the basis of the above given information, is the green eye colour a dominant or recessive trait? Justify your answer
b. What is the possible genetic makeup of Pooja’s brother’s eye colour?
c. What is the probability that the offspring of Pooja and Ravi will have green eyes? Also, show the inheritance of eye colour in the offspring with the help of a suitable cross.
OR
c. 50% of the offspring of Pooja’s brother are green eyed. With help of cross show how this is possible.
Chapter: [0.08] Heredity
 |
 |
The above images are that of a specialized slide projector. Slides are small transparencies mounted in sturdy frames ideally suited to magnification and projection since they have a very high resolution and a high image quality. There is a tray where the slides are to be put into a particular orientation so that the viewers can see the enlarged erect images of the transparent slides. This means that the slides will have to be inserted upside down in the projector tray.
To show her students the images of insects that she investigated in the lab, Mrs. Iyer brought a slide projector. Her slide projector produced 500 times enlarged and inverted image of a slide on a screen 10 m away.
a. Based on the text and data given in the above paragraph, what kind of lens must the slide projector have?
b. If v is the symbol used for image distance and u for object distance then with one reason state what will be the sign for `"𝑣"/"𝑢"` in the given case?
c. A slide projector has a convex lens with a focal length of 20 cm. The slide is placed upside down 21 cm from the lens. How far away should the screen be placed from the slide projector’s lens so that the slide is in focus?
OR
c. When a slide is placed 15 cm behind the lens in the projector, an image is formed 3 m in front of the lens. If the focal length of the lens is 14 cm, draw a ray diagram to show image formation. (not to scale)
Chapter: [0.09] Light - Reflection and Refraction
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 10 Science with solutions 2022 - 2023
Previous year Question paper for CBSE Class 10 Science-2023 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Science, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 10.
How CBSE Class 10 Question Paper solutions Help Students ?
• Question paper solutions for Science will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
