Advertisements
Advertisements
Question
On placing a copper coin in a test tube containing green ferrous sulphate solution, it will be observed that the ferrous sulphate solution ______.
Options
turns blue, and a grey substance is deposited on the copper coin.
turns colourless and a grey substance is deposited on the copper coin
turns colourless and a reddish–brown substance is deposited on the copper coin.
remains green with no change in the copper coin.
Solution
On placing a copper coin in a test tube containing green ferrous sulphate solution, it will be observed that the ferrous sulphate solution remains green with no change in the copper coin.
APPEARS IN
RELATED QUESTIONS
When acids react with metal, which gas is liberated?
Why do HCl, HNO3, etc., show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
10 mL of a solution of NaOH is found to be completely neutralised by 8 mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount of HCl solution (the same solution as before) required to neutralise it will be:
On adding dilute hydrochloric acid to copper oxide powder, the solution formed is blue-green.
On the basis of the above reaction, what can you say about the nature of copper oxide?
State the common and chemical names of the compound formed when plaster of Paris is mixed with water.
Read the following statements:
I. When a red litmus paper is dipped into reaction mixture of a saponification reaction, it turns blue and the reaction is exothermic.
II. When a blue litmus paper is dipped into reaction mixture of a saponification reaction, its colour does not change and the reaction is exothermic.
III. When a red litmus paper is dipped into reaction mixture of a saponification reaction, its colour does not change and the reaction is endothermic.
IV. When a blue litmus paper is dipped into reaction mixture of a saponification reaction, its colour does not change and the reaction is endothermic.
Which of the above statements are correct:
(A) I, and II
(B) II and III
(C) III and IV
(D) I and IV
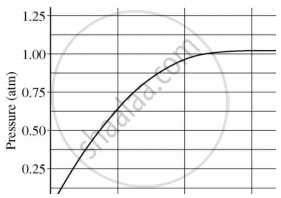
A student added 10 g of calcium carbonate in a rigid container, secured it tightly and started to heat it. After some time, an increase in pressure was observed, the pressure reading was then noted at intervals of 5 mins and plotted against time, in a graph as shown below. During which time interval did maximum decomposition take place?
Solutions of acids conduct ______.
What property do acids and bases have in common? Explain it with an example.
Give a balanced equation for the reaction:
Silver nitrate solution and sodium chloride solution.
