Advertisements
Advertisements
Question
Keerti added dilute Hydrochloric acid to four metals and recorded her observations as shown in the table given below:
| Metal | Gas Evolved |
| Copper | Yes |
| Iron | Yes |
| Magnesium | No |
| Zinc | Yes |
Select the correct observation(s) and give chemical equation(s) of the reaction involved.
Solution
\[\ce{Fe + HCl -> FeCl2/ FeCl3 + H2}\] (No deduction for balancing/ states)
\[\ce{Zn + HCl -> ZnCl2 + H2}\]
APPEARS IN
RELATED QUESTIONS
Give two examples of amphoteric oxides.
Name two metals which will displace hydrogen from dilute acids, and two metals which will not.
Give reason:
Sodium, potassium and lithium are stored under oil.
Which of the following oxide(s) of iron would be obtained on the prolonged reaction of iron with steam?
Which one of the following metals does not react with cold as well as hot water?
Silver articles become black on prolonged exposure to air. This is due to the formation of
If copper is kept open in air, it slowly loses its shining brown surface and gains a green coating. It is due to the formation of
Out of three metals, sodium, calcium and aluminium, which metal reacts most with water? Give the equation of reaction with all three metals.
Answer the questions based on the figure below:
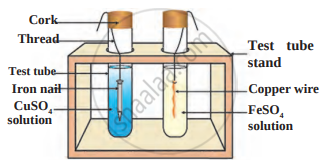
- Which experiment setup is demonstrated in the figure?
- What do you conclude after the reactions? Name reaction.
Metal oxides generally react with acids, but few oxides of metal also react with bases. Such metallic oxides are:
- MgO
- ZnO
- Al2O3
- Cao
