Advertisements
Advertisements
Question
Give two examples of amphoteric oxides.
Solution
- Aluminium oxide (Al2O3) and zinc oxide (ZnO) are examples of amphoteric oxides.
- The following reactions show the amphoteric character of zinc oxide:
- \[\ce{\underset{(Basic nature)}{ZnO_{(s)}} + 2HCl_{(aq)} -> \underset{Zinc chloride}{ZnCl2_{(aq)}} + H2O_{(l)}}\]
- \[\ce{\underset{(Acidic nature)}{ZnO_{(s)} + 2Na}OH_{(aq)} -> \underset{Sodium zincate}{Na2ZnO2_{(aq)}} + H2O_{(l)}}\]
APPEARS IN
RELATED QUESTIONS
Name two metals which will displace hydrogen from dilute acids, and two metals which will not.
What are amphoteric oxides?
Give reason:
Sodium, potassium and lithium are stored under oil.
Diamond : electric insulator : : _______ : electric conductor
Example of an amphoteric oxide is:
Which of the following metals liberate hydrogen with 5% HNO3?
(i) Cu
(ii) Zn
(iii) Mn
(iv) Mg
Generally, metals react with acids to give salt and hydrogen gas. Which of the following acids does not give hydrogen gas on reacting with metals (except Mn and Mg)?
Which of the following oxide(s) of iron would be obtained on prolonged reaction of iron with steam?
Silver articles become black on prolonged exposure to air. This is due to the formation of
Stainless steel is very useful material for our life. In stainless steel, iron is mixed with
When a metal X is treated with cold water, it gives a basic salt Y with molecular formula XOH (Molecular mass = 40) and liberates a gas Z which easily catches fire. Identify X, Y and Z and also write the reaction involved.
Metals generally react with dilute acids to produce hydrogen gas. Which one of the following metals does not react with dilute hydrochloric acid?
Which of the following reacts with cold water vigorously?
The metal which produces hydrogen gas on reaction with dilute hydrochloric acid as well as sodium hydroxide solution is
Material: Copper wire, Iron nail, Beaker or Big test tube.
Chemicals: Ferrous Sulphate and Copper Sulphate Solution.
- Which reaction you will study with the help of the above material and solutions? Draw the diagram of the experiment arrangement.
- How do you identify that the reaction is carried out?
- Which type of reaction did you observe?
Out of three metals, sodium, calcium and aluminium, which metal reacts most with water? Give the equation of reaction with all three metals.
Answer the questions based on the figure below:
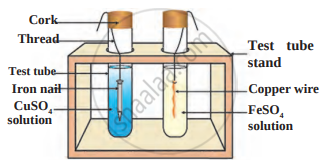
- Which experiment setup is demonstrated in the figure?
- What do you conclude after the reactions? Name reaction.
Answer the questions based on the figure below:
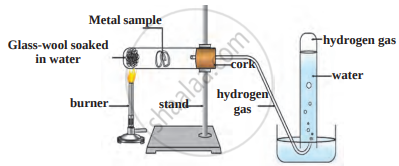
- Which reaction is shown in the figure?
- Which gas is evolved in the reaction?
- Give an example of reactants that rapidly show this reaction. Give equations.
- Give an example of reactants that do not react rapidly.
- In what condition will reactants of (c) part react? Give equation.
Keerti added dilute Hydrochloric acid to four metals and recorded her observations as shown in the table given below:
| Metal | Gas Evolved |
| Copper | Yes |
| Iron | Yes |
| Magnesium | No |
| Zinc | Yes |
Select the correct observation(s) and give chemical equation(s) of the reaction involved.
Metal oxides generally react with acids, but few oxides of metal also react with bases. Such metallic oxides are:
- MgO
- ZnO
- Al2O3
- Cao
Write a chemical equation showing the ionic products formed on dissolving potassium hydroxide in water.
On adding dilute sulphuric acid to a test tube containing a metal ‘X’, a colourless gas is produced when a burning match stick is brought near it. Which of the following correctly represents the metal ‘X’?
