Advertisements
Advertisements
Question
Give reason:
Sodium, potassium and lithium are stored under oil.
Solution
- All three metals react with water, producing a lot of heat. As a result, hydrogen evolves and catches fire.
- They cannot be kept in the air because air contains moisture or water vapours. They are kept under kerosene to avoid contact with both air and water.
RELATED QUESTIONS
What are amphoteric oxides?
Diamond : electric insulator : : _______ : electric conductor
Which of the following oxide(s) of iron would be obtained on the prolonged reaction of iron with steam?
Example of an amphoteric oxide is:
Generally, metals react with acids to give salt and hydrogen gas. Which of the following acids does not give hydrogen gas on reacting with metals (except Mn and Mg)?
Which of the following oxide(s) of iron would be obtained on prolonged reaction of iron with steam?
Stainless steel is very useful material for our life. In stainless steel, iron is mixed with
When a metal X is treated with cold water, it gives a basic salt Y with molecular formula XOH (Molecular mass = 40) and liberates a gas Z which easily catches fire. Identify X, Y and Z and also write the reaction involved.
Which of the following reacts with cold water vigorously?
The metal which produces hydrogen gas on reaction with dilute hydrochloric acid as well as sodium hydroxide solution is
Zinc sulphate forms a colourless solution in water. Will you observe any colour on adding copper turning in it?
Material: Copper wire, Iron nail, Beaker or Big test tube.
Chemicals: Ferrous Sulphate and Copper Sulphate Solution.
- Which reaction you will study with the help of the above material and solutions? Draw the diagram of the experiment arrangement.
- How do you identify that the reaction is carried out?
- Which type of reaction did you observe?
Out of three metals, sodium, calcium and aluminium, which metal reacts most with water? Give the equation of reaction with all three metals.
Answer the questions based on the figure below:
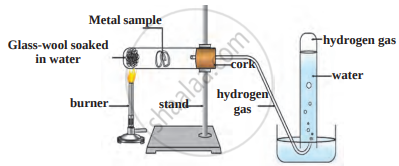
- Which reaction is shown in the figure?
- Which gas is evolved in the reaction?
- Give an example of reactants that rapidly show this reaction. Give equations.
- Give an example of reactants that do not react rapidly.
- In what condition will reactants of (c) part react? Give equation.
Keerti added dilute Hydrochloric acid to four metals and recorded her observations as shown in the table given below:
| Metal | Gas Evolved |
| Copper | Yes |
| Iron | Yes |
| Magnesium | No |
| Zinc | Yes |
Select the correct observation(s) and give chemical equation(s) of the reaction involved.
Metal oxides generally react with acids, but few oxides of metal also react with bases. Such metallic oxides are:
- MgO
- ZnO
- Al2O3
- Cao
Write a chemical equation showing the ionic products formed on dissolving potassium hydroxide in water.
On adding dilute sulphuric acid to a test tube containing a metal ‘X’, a colourless gas is produced when a burning match stick is brought near it. Which of the following correctly represents the metal ‘X’?
