Advertisements
Advertisements
Question
On adding dilute sulphuric acid to a test tube containing a metal ‘X’, a colourless gas is produced when a burning match stick is brought near it. Which of the following correctly represents the metal ‘X’?
Options
Sodium
Sulphur
Copper
Silver
Solution
Sodium
Explanation:
The element X is sodium. When sodium metal reacts with sulphuric acid, sodium replaces the hydrogen ion, forming sodium sulphate and hydrogen gas. When a lit matchstick is brought close to the mouth of the hydrogen gas-filled test tube, it burns with a pop-up sound.
\[\ce{H2SO4 + 2Na -> Na2SO4 + H2}\]
APPEARS IN
RELATED QUESTIONS
Diamond : electric insulator : : _______ : electric conductor
Which of the following oxide(s) of iron would be obtained on the prolonged reaction of iron with steam?
Example of an amphoteric oxide is:
Which of the following oxide(s) of iron would be obtained on prolonged reaction of iron with steam?
When a metal X is treated with cold water, it gives a basic salt Y with molecular formula XOH (Molecular mass = 40) and liberates a gas Z which easily catches fire. Identify X, Y and Z and also write the reaction involved.
Which of the following reacts with cold water vigorously?
Out of three metals, sodium, calcium and aluminium, which metal reacts most with water? Give the equation of reaction with all three metals.
Answer the questions based on the figure below:
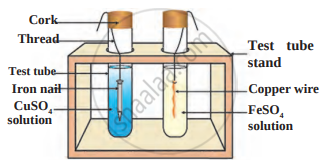
- Which experiment setup is demonstrated in the figure?
- What do you conclude after the reactions? Name reaction.
Keerti added dilute Hydrochloric acid to four metals and recorded her observations as shown in the table given below:
| Metal | Gas Evolved |
| Copper | Yes |
| Iron | Yes |
| Magnesium | No |
| Zinc | Yes |
Select the correct observation(s) and give chemical equation(s) of the reaction involved.
Write a chemical equation showing the ionic products formed on dissolving potassium hydroxide in water.
