English Medium
Academic Year: 2023-2024
Date: March 2024
Duration: 3h
Advertisements
General Instructions:
- This question paper consists of 39 questions in 5 sections.
- All questions are compulsory. However, an internal choice is provided in some questions. Students are expected to attempt only one of these questions.
- Section A consists of 20 objective type questions carrying 1 mark each. Q. No. 17 to 20 are Assertion - Reasoning based questions.
- Section B consists of 6 Very Short questions carrying 02 marks each. Answers to these questions should be in the range of 30 to 50 words.
- Section C consists of 7 Short Answer type questions carrying 03 marks each. Answers to these questions should be in the range of 50 to 80 words
- Section D consists of 3 Long Answer type questions carrying 05 marks each. Answers to these questions should be in the range of 80 to 120 words.
- Section E consists of 3 source-based/case-based units of assessment of 04 marks each with sub-parts.
\[\ce{Fe2O3 + 2Al -> Al2O3 + 2Fe}\]
The above reaction is an example of a ______.
combination reaction
double displacement reaction
decomposition reaction
displacement reaction
Chapter: [0.01] Chemical Reactions and Equations
10 mL of a solution of NaOH is found to be completely neutralised by 8 mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount of HCl solution (the same solution as before) required to neutralise it will be:
4 mL
8 mL
12 mL
16 mL
Chapter: [0.02] Acids, Bases and Salts
Food cans are coated with tin and not with zinc because ______.
zinc is costlier than tin
zinc has a higher melting point than tin
zinc is more reactive than tin
zinc is less reactive than tin
Chapter: [0.03] Metals and Non Metals
Butanone is a four-carbon compound with the functional group:
carboxylic acid
aldehyde
ketone
alcohol
Chapter: [0.03] Metals and Non Metals [0.04] Carbon and its Compounds
The xylem in plants is responsible for ______.
transport of water
transport of food
transport of amino acids
transport of oxygen
Chapter: [0.05] Life Processes
Which of the following terms does not represent electrical power in a circuit?
I2R
IR2
VI
`"V"^2/"R"`
Chapter: [0.11] Electricity
Which of the following is not a part of the female reproductive system in human beings?
Ovary
Uterus
Vas deferens
Fallopian tube
Chapter: [0.07] How do Organisms Reproduce?
A Mendelian experiment consisted of breeding tall pea plants bearing violet flowers with short pea plants bearing white flowers. The progeny all bore violet flowers, but almost half of them were short. This suggests that the genetic make-up of the tall parent can be depicted as:
TTWW
TTww
TtWW
TtWw
Chapter: [0.08] Heredity
Which of the following lenses would you prefer to use while reading small letters found in a dictionary?
A convex lens of focal length 50 cm.
A concave lens of focal length 50 cm.
A convex lens of focal length 5 cm.
A concave lens of focal length 5 cm.
Chapter: [0.09] Light - Reflection and Refraction
The human eye can focus objects at different distances by adjusting the focal length of the eye lens. This is due to ______.
presbyopia
accommodation
near-sightedness
far-sightedness
Chapter: [0.1] The Human Eye and the Colourful World
A piece of wire of resistance R is cut into five equal parts. These parts are then connected in parallel. If the equivalent resistance of this combination is R’, then the ratio `"R"/"R'"` is ______.
`1/25`
`1/5`
5
25
Chapter: [0.11] Electricity
Which of the following correctly describes the magnetic field near a long straight wire?
The field consists of straight lines perpendicular to the wire
The field consists of straight lines parallel to the wire
The field consists of radial lines originating from the wire
The field consists of concentric circles centred on the wire
Chapter: [0.12] Magnetic Effects of Electric Current
Which of the following constitute a food-chain?
Grass, wheat and mango
Grass, goat and human
Goat, cow and elephant
Grass, fish and goat
Chapter: [0.13] Our Environment
A spherical mirror and a thin spherical lens have each a focal length of -15 cm. The mirror and the lens are likely to be ______.
both concave
both convex
the mirror is concave and the lens is convex
the mirror is convex, but the lens is concave
Chapter: [0.09] Light - Reflection and Refraction
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that ______.
The food is not cooked completely
The fuel is not burning completely
The fuel is wet
The fuel is burning completely
Chapter:
Which of the following is a plant hormone?
Insulin
Thyroxin
Oestrogen
Cytokinin
Chapter: [0.06] Control and Co-ordination
Assertion: Height in pea plants is controlled by the efficiency of enzymes and is thus genetically controlled.
Reason: Cellular DNA is the information source for making proteins in the cell.
Both Assertion and Reason are true and Reason is the correct explanation of Assertion.
Both Assertion and Reason are true and Reason is not the correct explanation of Assertion.
Assertion is true but Reason is false.
Assertion is False but Reason is true.
Chapter: [0.08] Heredity
- Assertion: A compass needle is placed near a current-carrying wire. The deflection of the compass needle decreases when the magnitude of the current in the wire is increased.
- Reason: The strength of a magnetic field at a point near the conductor increases by increasing the current.
Both A and R are true, and R is the correct explanation of A.
Both A and R are true, and R is not the correct explanation of A.
A is true but R is false.
A is false but R is true.
Chapter: [0.12] Magnetic Effects of Electric Current
Assertion (A): The inner walls of the small intestine have finger like projections called villi which are rich in blood.
Reason (R): These villi have large surface area to help the small intestine in completing the digestion of food.
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true, but (R) is false.
(A) is false, but (R) is true.
Chapter: [0.05] Life Processes
Assertion (A): The energy which passes to the herbivores does not come back to autotrophs.
Reason (R): The flow of energy in a food chain is unidirectional.
Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of (A).
Both Assertion (A) and Reason (R) are true but Reason (R) is not the correct explanation of (A).
Assertion (A) is true, but Reason (R) is false.
Assertion (A) is false, but Reason (R) is true.
Chapter: [0.13] Our Environment
What do you mean by a precipitation reaction? Explain by giving examples.
Chapter: [0.01] Chemical Reactions and Equations
Five solutions, A, B, C, D and E, when tested with universal indicator, showed pH as 4, 1, 11, 7 and 9, respectively. Which solution is
- neutral?
- strongly alkaline?
- strongly acidic?
- weakly acidic?
- weakly alkaline?
Arrange the pH in increasing order of hydrogen-ion concentration.
Chapter: [0.02] Acids, Bases and Salts
Advertisements
What are outside raw materials used for by an organism?
Chapter: [0.05] Life Processes
How are oxygen and carbon dioxide transported in human beings?
Chapter: [0.05] Life Processes [0.05] Life Processes
Give reason for the following:
Vegetative propagation is practised for growing only some type of plants.
Chapter: [0.07] How do Organisms Reproduce?
The far point of a myopic person is 80 cm in front of the eye. What is the nature and power of the lens required to correct the problem?
Chapter: [0.1] The Human Eye and the Colourful World
Explain why the planets do not twinkle.
Chapter: [0.1] The Human Eye and the Colourful World
A man went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset, but after a futile argument, the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Chapter: [0.03] Metals and Non Metals
Write the balanced chemical equation for the following reaction.
\[\ce{Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water}\]
Chapter: [0.01] Chemical Reactions and Equations
Write the balanced chemical equation for the following reaction.
\[\ce{Aluminium + Copper chloride -> Aluminium chloride + Copper}\]
Chapter: [0.01] Chemical Reactions and Equations
Write the balanced chemical equation for the following and identify the type of reaction.
\[\ce{Potassium bromide (aq) + Barium iodide (aq) -> Potassium iodide (aq) + Barium bromide (s)}\]
Chapter: [0.01] Chemical Reactions and Equations
Consider the following salt:
YCl
What would be the pH of the solution if, in YCl, Y is sodium? Give a reason for your answer.
Chapter: [0.02] Acids, Bases and Salts
Consider the following salt:
NH4X
If in salt NH4X, X is nitrate, then its solution will give what colour with a universal indicator? Why?
Chapter: [0.02] Acids, Bases and Salts
Consider the following salt:
\[\ce{ZCO3}\]
What would be the change in colour in blue litmus if \[\ce{ZCO3}\] is added to it and Z is potassium?
Chapter: [0.02] Acids, Bases and Salts
A metal 'M' on reacting with dilute acid liberates a gas 'G'. The same metal also liberates gas 'G' when reacts with a base.
- Write the name of gas 'G'.
- How will you test the presence of this gas?
- Write chemical equations for the reactions of the metal with (1) an acid and (2) a base.
Chapter: [0.02] Acids, Bases and Salts
Describe the structure and functioning of nephrons.
Chapter: [0.05] Life Processes [0.05] Life Processes
Why is DNA copying an essential part of the process of reproduction?
Chapter: [0.07] How do Organisms Reproduce?
How is the process of pollination different from fertilization?
Chapter: [0.07] How do Organisms Reproduce?
Advertisements
A person needs a lens of power -5.5 dioptres for correcting his distant vision. For correcting his near vision he needs a lens of power +1.5 dioptre. What is the focal length of the lens required for correcting (i) distant vision and (ii) near vision?
Chapter: [0.1] The Human Eye and the Colourful World
Give reasons:
Platinum, gold and silver are used to make jewellery.
Chapter: [0.01] Chemical Reactions and Equations [0.03] Metals and Non Metals
Give reason:
Sodium, potassium and lithium are stored under oil.
Chapter: [0.03] Metals and Non Metals
Give reasons.
Aluminium is a highly reactive metal, yet it is used to make utensils for cooking.
Chapter: [0.03] Metals and Non Metals
If a harmful chemical enters a food chain comprising peacocks, plants, rats and snakes, which of these organisms is likely to have the highest concentration of the chemical in its body? Justify your answer. Name the process involved and define it.
Chapter: [0.13] Our Environment
What is the role of the brain in reflex action?
Chapter: [0.06] Control and Co-ordination
What happens at the synapse between two neurons?
Chapter: [0.06] Control and Co-ordination
Three incandescent bulbs of 100 W each are connected in series in an electric circuit. In another circuit another set of three bulbs of the same wattage are connected in parallel to the same source.
- Will the bulb in the two circuits glow with the same brightness? Justify your answer.
- Now let one bulb in both the circuits get fused. Will the rest of the bulbs continue to glow in each circuit? Give reason.
Chapter: [0.11] Electricity
Redraw the circuit of question 1, putting in an ammeter to measure the current through the resistors and a voltmeter to measure the potential difference across the 12 Ω resistors. What would be the readings in the ammeter and the voltmeter?
Chapter: [0.11] Electricity
Write the chemical equation for the following:
Combustion of methane
Chapter: [0.04] Carbon and its Compounds
Write the chemical equation for the following:
Oxidation of ethanol
Chapter: [0.04] Carbon and its Compounds
Write the chemical equation for the following:
Hydrogenation of ethene
Chapter: [0.04] Carbon and its Compounds
Write the chemical equation for the following:
Esterification Reaction
Chapter: [0.04] Carbon and its Compounds
Write the chemical equation for the following:
Saponification Reaction
Chapter: [0.04] Carbon and its Compounds
Describe in brief the cleansing action of soap.
Chapter: [0.04] Carbon and its Compounds
Sex of an individual is determined by different factors in various species. Some animals rely entirely on the environmental ones, while in some other animals the individuals can change their sex during their life time indicating that sex of some species is not genetically determined. However in human beings, the sex of an individual is largely determined genetically.
- In what way are the sex chromosomes 'X' and 'Y' different in size? Name the mismatched pair of sex chromosome in humans.
- Write the number of pair/pairs of sex chromosomes present in human beings. In which one of the parent (male/female) perfect pair/pairs of sex chromosome are present?
- Citing two examples, justify the statement "Sex of an individual is not always determined genetically".
OR
Draw a flow chart to show that sex is determined genetically in human beings.
Chapter: [0.08] Heredity
A student took three concave mirrors of different focal lengths and performed the experiment to see the image formation by placing an object at different distance with these mirrors as shown in the following table.
| Case No. | Object-distance | Focal length |
| I | 45 cm | 20 cm |
| II | 30 cm | 15 cm |
| III | 20 cm | 30 cm |
Now answer the following questions:
(a) List two properties of the image formed in Case I.
(b) In which one of the cases given in the table, the mirror will form real image of same size and why?
(c) Name the type of mirror used by dentists. Given reason why do they use such type of mirrors.
OR
(c) Look at the table and identify the situation (object distance and focal length) which resembles the situation in which concave mirrors are used as shaving mirrors? Draw a ray diagram to show the image formation in this case.
Chapter: [0.09] Light - Reflection and Refraction
A student was asked to perform an experiment to study the force on a current carrying conductor in a magnetic field. He took a small aluminium rod AB, a strong horse shoe magnet, some connecting wires, a battery and a switch and connected them as shown. He observed that on passing current, the rod gets displaced. On reversing the direction of current, the direction of displacement also gets reversed. On the basis of your understanding of this phenomenon, answer the following questions:
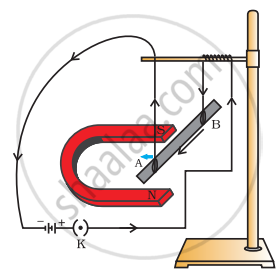
- Why does the rod get displaced on passing current through it?
- State the rule that determines the direction of the force on the conductor AB.
-
- If the U-shaped magnet is held vertically and the aluminium rod is suspended horizontally with its end B towards due north, then on passing current through the rod from B to A as shown, in which direction will the rod be displaced?
- Name any two devices that use current carrying conductors and magnetic fields.
OR
Draw the pattern of magnetic field lines produced around a current carrying straight conductor held vertically on a horizontal cardboard. Indicate the direction of the field lines as well as the direction of current flowing through the conductor.
Chapter: [0.12] Magnetic Effects of Electric Current
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 10 Science with solutions 2023 - 2024
Previous year Question paper for CBSE Class 10 Science-2024 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Science, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 10.
How CBSE Class 10 Question Paper solutions Help Students ?
• Question paper solutions for Science will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
