Advertisements
Advertisements
Question
Give one example of a decomposition reaction which is carried out by applying heat.
Solution
Example of the decomposition reaction are as follows:
by applying heat:
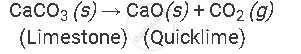
APPEARS IN
RELATED QUESTIONS
What type of chemical reaction take place when silver bromide is exposed to sunlight?
What type of chemical reaction take place when electricity is passed through water?
What type of reaction is represented by the following equation?
NH4NO2 → N2 + 2H2O
Name the product formed on strongly heating ferrous sulphate crystals. What type of chemical reaction occurs in this change?
Explain the following type of chemical reaction, giving two examples for it:
Decomposition reaction
What do you mean by redox reaction ? Explain with the help of an example.
How can the rate of the chemical reaction, namely, decomposition of hydrogen peroxide be increased?
Differentiate between direct combination reaction and a decomposition reaction.
Differentiate between the following:
Electrolytic decomposition and photochemical decomposition.

- Identify the gasses evolved at the anode and cathode in the above experimental set up.
- Name the process that occurs. Why is it called so?
- Illustrate the reaction of the process with the help of a chemical equation.
