Advertisements
Advertisements
Question
Differentiate between the following:
Electrolytic decomposition and photochemical decomposition.
Solution
- Electrolytic decomposition: “A decomposition reaction which is brought about by the passage of electric current.”
- Photochemical decomposition: “A decomposition reaction which takes place in presence of light.”
e.g. decomposition of silver salts in the presence of sunlight.
APPEARS IN
RELATED QUESTIONS
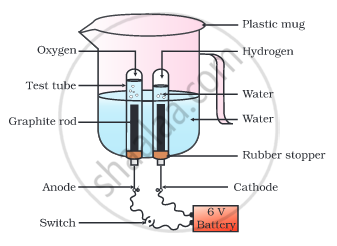
Why is the amount of gas collected in one of the test tubes in the following Activity double of the amount collected in the other? Name this gas.
- Take a plastic mug. Drill two holes at its base and fit rubber stoppers in these holes. Insert carbon electrodes in these rubber stoppers as shown in the following Fig.
- Connect these electrodes to a 6 volt battery.
- Fill the mug with water such that the electrodes are immersed. Add a few drops of dilute sulphuric acid to the water.
- Take two test tubes filled with water and invert them over the two carbon electrodes.
- Switch on the current and leave the apparatus undisturbed for some time.
- You will observe the formation of bubbles at both the electrodes. These bubbles displace water in the test tubes.
- Is the volume of the gas collected the same in both the test tubes?
• Once the test tubes are filled with the respective gases, remove them carefully. - Test these gases one by one by bringing a burning candle close to the mouth of the test tubes.
Caution: This step must be performed carefully by the teacher.
- What happens in each case?
- Which gas is present in each test tube?
The white solid compound A decomposes quite rapidly on heating in the presence of a black substance X to form a solid compound B and a gas C. When an aqueous solution of compound B is reacted with silver nitrate solution, then a white precipitate of silver chloride is obtained along with potassium nitrate solution. Gas C does not burn itself but helps burn other things.
(a) What is compound A?
(b) What is compound B?
(c) What is gas C?
(d) What do you think is the black substance X? What is its function?
(e) What is the general name of substances like X?
Classify the following reaction as combination, decomposition, displacement, precipitation and neutralization. Also balance the equation.
Identify the type of following reaction :
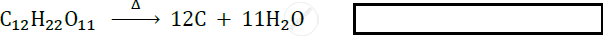
Answer the following question.
2 g of silver chloride is taken in a china dish and the china dish is placed in sunlight for some time. What will be your observation in this case? Write the chemical reaction involved in the form of a balanced chemical equation. Identify the type of chemical reaction.
Classify the following reaction into different type:
Differentiate between direct combination reaction and a decomposition reaction.
Classify the following reaction into –
- Direct combination
- Decomposition
- Displacement
- Double decomposition
The reaction is – Zinc hydroxide on heating gives zinc oxide and water.
Differentiate between the following:
Thermal decomposition and thermal dissociation.
Complete the following reaction:
