Advertisements
Advertisements
Question
The white solid compound A decomposes quite rapidly on heating in the presence of a black substance X to form a solid compound B and a gas C. When an aqueous solution of compound B is reacted with silver nitrate solution, then a white precipitate of silver chloride is obtained along with potassium nitrate solution. Gas C does not burn itself but helps burn other things.
(a) What is compound A?
(b) What is compound B?
(c) What is gas C?
(d) What do you think is the black substance X? What is its function?
(e) What is the general name of substances like X?
Solution
(a) Compound A is potassium chlorate (KClO3).
(b) Compound B is potassium chloride (KCl).
(c) Gas C is oxygen gas (O2), which does not burn itself but helps burn other things.
(d) Black substance X is manganese oxide (MnO2). Its function is to catalyse the reaction.
(e) General name of substance like X (MnO2) is catalyst.
APPEARS IN
RELATED QUESTIONS
What does one mean by exothermic reaction? Give example.
State an important use of decomposition reactions.
Which of the following can be decomposed by the action of light?
(a) NaCl
(b) KCl
(c) AgCl
(d) CuCl
A metal salt MX when exposed to light splits up to form metal M and a gas X2. Metal M is used in making ornaments whereas gas X2 is used in making bleaching powder. The salt MX is itself used in black and white photography.
(a) What do you think metal M is?
(b) What could be gas X2?
(c) Name the metal salt MX.
(d) Name any two salt solutions which on mixing together can produce a precipitate of salt MX.
(e) What type of chemical reaction takes place when salt MX is exposed to light? Write the equation of the reaction?
How can the rate of the chemical reaction, namely, decomposition of hydrogen peroxide be increased?
Differentiate between the following:
Electrolytic decomposition and photochemical decomposition.
Photolysis is a decomposition reaction caused by ______
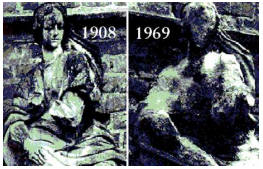
Marble statues are corroded or stained rain water. Identify the main reason.
Balance the following chemical equation and identify the type of chemical reaction.
`"HgO"("s") overset("(heat)")(->) "Hg"("l") + "O"_2("g")`
Complete the following reaction:
\[\ce{C_12H_22O11->[Heat]}\] ______ + ______.
