Advertisements
Online Mock Tests
Chapters
2: Acids, Bases and Salts
3: Metals and Non-metals
4: Carbon And Its Compounds
5: Periodic Classification Of Elements
![Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 chapter 1 - Chemical Reactions and Equations Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 chapter 1 - Chemical Reactions and Equations - Shaalaa.com](/images/9352831810-chemistry-science-english-class-10_6:7eeaf5db0f4a49c580d3c8746ff5ab36.jpg)
Advertisements
Solutions for Chapter 1: Chemical Reactions and Equations
Below listed, you can find solutions for Chapter 1 of CBSE Lakhmir Singh for Chemistry (Science) [English] Class 10.
Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 1 Chemical Reactions and Equations Exercise 1 [Pages 18 - 23]
Why is respiration considered an exothermic reaction? Explain.
On what basis is a chemical equation balanced?
What happens chemically when quicklime is added to water filled in a bucket?
Why should a magnesium ribbon be cleaned before it is burnt in air?
State whether the following statement is true or false:
A chemical equation can be balanced easily by altering the formula of a reactant or product.
What is wrong with the following chemical equation?
Mg + O → MgO
Correct and balance it.
What does the symbol (aq) represent in a chemical equation?
Why is photosynthesis considered an endothermic reaction?
How will you indicate a solution made in water in a chemical equation?
How will you indicate Exothermic reaction in a chemical equation?
How will you indicate Endothermic reaction in a chemical equation?
Translate the following statement into chemical equation and then balance it.
Hydrogen sulphide gas burns in the air to give water and sulphur dioxide.
Translate the following statement into chemical equation and then balance the equation:
Phosphorus burns in oxygen to give phosphorus pentoxide.
Translate the following statement into chemical equation and then balance the equation:
Carbon disulphide burns in air to give carbon dioxide and sulphur dioxide.
Translate the following statement into chemical equation and then balance the equation:
Aluminium metal replaces iron from ferric oxide, Fe2O3, giving aluminium oxide and iron.
Translate the following statement into chemical equation and then balance the equation:
Barium chloride reacts with zinc sulphate to give zinc chloride and barium sulphate.
Write the balanced chemical equation for the following reaction.
\[\ce{Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water}\]
Write the balanced chemical equation for the following reaction.
\[\ce{Aluminium + Copper chloride -> Aluminium chloride + Copper}\]
Complete and balance the following equation:
NaOH + ............  Na2 SO4 + H2O
Na2 SO4 + H2O
Complete and balance the following equation:
CA (OH)2 + .............  CaCO3 + H2O
CaCO3 + H2O
Correct and balance the following equation:
Ca + H2O  CaOH + H
CaOH + H
Correct and balance the following equation:
N + H  NH3
NH3
Write complete balanced equation for the following reaction:
Calcium (solid) + water (liquid)  Calcium Hydroxide (solution) + Hydrogen (gas)
Calcium Hydroxide (solution) + Hydrogen (gas)
Write complete balanced equation for the following reaction:
Sulphur dioxide (gas) + Oxgyen (gas)  Sulphur trioxide (gas)
Sulphur trioxide (gas)
Balance the following equation:
Na + O2  Na2O
Na2O
Balance the given equation:
H2O2  H2O + O2
H2O + O2
Balance the given equation:
Mg(OH)2 + HCI MgCI + H2O
Balance the given equation:
Fe + O2  Fe2O3
Fe2O3
Balance the given equation:
AI(OH)3  AI2O3 +H2O
AI2O3 +H2O
Balance the given equation:
NH3 + CuO  Cu +N2 +H2O
Cu +N2 +H2O
Balance the given equation:
AI2(SO4)3 +NaOH  AI(OH)3 + Na2SO4
AI(OH)3 + Na2SO4
Balance the given equation:
HNO3 + Ca(OH)2  Ca(NO3)2 + H2O
Ca(NO3)2 + H2O
Balance the gievn equation:
NaOH + H2SO4  Na2SO4 + H2O
Na2SO4 + H2O
Balance the given equation:
BaCI2 + H2SO4  BaSO4 + HCI
BaSO4 + HCI
Fill in the following blank with suitable word:
Chemical equations are balanced to satisfy the law of ............
Fill in the following blank with suitable word:
A solution made in water is known as an ........... solution and indicated by the symbol ...........
Give one example of a chemical reaction.
State two characteristics of the chemical reaction which takes place when dilute sulphuric acid is poured over zinc granules.
Give two characteristics of the chemical reaction which occurs on adding potassium iodide solution to lead nitrate solution.
What is a chemical equation? Explain with the help of an example.
Giving examples, state the difference between balanced and unbalanced chemical equations.
Balance the given chemical equation:
NH3 → N2 + H2
Balance the given chemical equation:
C + CO2 → CO
When hydrogen is passed over copper oxide, copper and steam are formed. Write a balanced equation for this reaction and state which of the chemicals are elements.
When hydrogen is passed over copper oxide, copper and steam are formed. Write a balanced equation for this reaction and state which of the chemicals are compounds.
When hydrogen is passed over copper oxide, copper and steam are formed. Write a balanced equation for this reaction and state which of the chemicals are reactants.
When hydrogen is passed over copper oxide, copper and steam are formed. Write a balanced equation for this reaction and state which of the chemicals are products.
When hydrogen is passed over copper oxide, copper and steam are formed. Write a balanced equation for this reaction and state which of the chemicals are metals.
When hydrogen is passed over copper oxide, copper and steam are formed. Write a balanced equation for this reaction and state which of the chemicals are non-metals.
What are the various ways in which a chemical equation can be made more informative? Give examples to illustrate your answer.
Write balanced chemical equation from the following information:
An aqueous calcium hydroxide solution (lime water) reacts with carbon dioxide gas to produce a solid calcium carbonate precipitate and water.
What is a balanced chemical equation? Why should a chemical equation be balanced?
Aluminium burns in chlorine to form aluminium chloride (AlCl3). Write a balanced chemical equation for this reaction.
Translate the following statement into chemical equation and then balance it.
Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Explain, with example, how the physical states of the reactants and products can be shown in a chemical equation.
Balance the following equation and add state symbols:
Zn + HCI → ZnCI2 + H2
Convey the following information in the form of a balanced chemical equation:
"An aqueous solution of ferrous sulphate reacts with an aqueous solution of sodium hydroxide to form a precipitate of ferrous hydroxide and sodium sulphate remains in solution."
Write any two observations in an activity which may suggest that a chemical reaction has taken place. Give an example in support of your answer.
Aluminium hydroxide reacts with sulphuric acid to form aluminium sulphate and water. Write a balanced equation for this reaction.
Balance the following chemical equation:
MnO2 + HCI → MnCI2 + CI2 + H2O
Write the balanced equations for the following reaction, and add the state symbols:
Magnesium carbonate reacts with hydrochloric acid to produce magnesium chloride, carbon dioxide and water.
Write the balanced equation for the following reaction, and add the state symbols:
Sodium hydroxide reacts with sulphuric acid to produce sodium sulphate and water.
Carbon monoxide reacts with hydrogen under certain conditions to form methanol (CH3OH). Write a balanced chemical equation for this reaction indicating the physical states of reactants and product as well as the conditions under which this reaction takes place.
Potassium chlorate (KClO3) on heating forms potassium chloride and oxygen. Write a balanced equation for this reaction and indicate the evolution of gas.
Rewrite the following information in the form of a balanced chemical equation:
Magnesium burns in carbon dioxide to form magnesium oxide and carbon.
Substitute formulae for names and balance the following equation:
Calcium carbonate reacts with hydrochloric acid to produce calcium chloride, water and carbon dioxide gas.
Write balanced chemical equation with state symbols for the following reaction:
Sodium hydroxide solution reacts with hydrochloric acid solution to produce sodium chloride solution and water.
Ammonia reacts with oxygen to form nitrogen and water. Write a balanced chemical equation for this reaction. Add the state symbols for all the reactants and products.
Write a balanced chemical equation for the process of photosynthesis giving the physical states of all the substances involved and the conditions of the reaction.
Translate the following statement into chemical equation and then balance it.
Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
When potassium nitrate is heated, it decomposes into potassium nitrite and oxygen. Write a balanced equation for this reaction and add the state symbols of the reactants and products.
What is meant by chemical reaction? Explain with the help of an example.
With the help of an appropriate example, justify that some of the chemical reactions are determined by Evolution of a gas.
Give chemical equation for the reaction involved in the above case.
With the help of an appropriate example, justify that some of the chemical reactions are determined by Change in colour
Give chemical equation for the reaction involved in the above case.
Give one example of a chemical reaction characterised by formation of a precipitate.
With the help of an appropriate example, justify that some of the chemical reactions are determined by Change in temperature.
Give chemical equation for the reaction involved in the above case.
Give one example of a chemical reaction characterised by change in state.
State the various characteristics of chemical reactions.
State one characteristic of the chemical reaction which takes place when dilute hydrochloric acid is added to sodium carbonate.
State one characteristic of the chemical reaction which takes place when lemon juice is added gradually to potassium permanganate solution.
State one characteristic of the chemical reaction which takes place when dilute sulphuric acid is added to barium chloride solution.
State one characteristic of the chemical reaction which takes place when quicklime is treated with water
State one characteristic of the chemical reaction which takes place when wax is burned in the form of a candle?
What do you understand by exothermic reactions?
What do you understand by endothermic reactions?
Give one example of an exothermic reaction.
Give one example of an endothermic reaction.
Is Burning of natural gas an endothermic reaction or an exothermic reaction?
Is Photosynthesis an endothermic reaction or an exothermic reaction?
Is Electrolysis of water an endothermic reaction or an exothermic reaction?
Is Respiration an endothermic reaction or an exothermic reaction?
Is Decomposition of calcium carbonate an endothermic reaction or an exothermic reaction?
One of the following does not happen during a chemical reaction. This is:
(a) Breaking of old chemical bonds and formation of new chemical bonds
(b) Formation of new substances with entirely different properties
(c) Atoms of one element change into those of another element to form new products.
(d) A rearrangement of atoms takes place to form new products.
Which of the following does not involve a chemical reaction?
digestion of food in our body
process of respiration
burning of candle wax when heated
melting of candle wax on heating
You are given the solution of lead nitrate. In order to obtain a yellow precipitate you should mix with it a solution of:
(a) potassium chloride
(b) potassium nitride
(c) potassium sulphide
(d) potassium iodide
An acid which can decolourise purple coloured potassium permanganate solution is:
(a) sulphuric acid
(b) citric acid
(c) carbonic acid
(d) hydrochloric acid
The chemical reaction between two substances is characterised by a change in colour from orange to green. These two substances are most likely to be:
(a) potassium dichromate solution and sulphur dioxide
(b) potassium permanganate solution and sulphur dioxide
(c) potassium permanganate solution and lemon juice
(d) potassium dichromate solution and carbon dioxide.
The chemical reaction between quicklime and water is characterised by:
(a) evolution of hydrogen gas
(b) formation of slaked lime precipitate
(c) change in temperature of mixture
(d) change in colour of the product
One of the following is an endothermic reaction. This is:
(a) combination of carbon and oxygen to form carbon monoxide
(b) combination of nitrogen and oxygen to form nitrogen monoxide
(c) combination of glucose and oxygen to form carbon dioxide and water
(d) combination of zinc and hydrochloric acid to form zinc chloride and hydrogen
Which of the following is not an endothermic reaction?
(a) CaCO3 → CaO + CO2
(b) 2H2O →2H2 + O2
(c) 6CO2 + 6H2O → C6H12O6 + 6O2
(d) C6H12O6 + 6O2 → 6CO2 + 6H2O
One of the following is an exothermic reaction. This is:
(a) electrolysis of water
(b) conversion of limestone into quicklime
(c) process of respiration
(d) process of photosynthesis
The chemical equations are balanced to satisfy one of the following laws in chemical reactions. This law is know as:
(a) law of conservation of momentum
(b) law of conservation of mass
(c) law of conservation of motion
(d) law of conservation of magnetism
When the solution of substance X is added to a solution of potassium iodide, then a yellow solid separates out from the solution.
(a) What do you think substance X is likely to be?
(b) Name the substance which the yellow solid consists of.
(c) Which characteristic of chemical reaction is illustrated by this example?
(d) Write a balanced chemical equation for the reaction which takes place. Mention the physical states of all the reactant and products involved in the chemical equation.
When water is added gradually to a white solid X, a hissing sound is heard and a lot of heat is produced forming a product Y. A suspension of Y in water is applied to the walls of a house during white washing. A clear solution of Y is also used for testing carbon dioxide gas in the laboratory.
(a) What could be solid X? Write its chemical formula.
(b) What could be product Y? Write its chemical formula.
(c) What is the common name of the solution of Y which is used for testing carbon dioxide gas?
(d) Write chemical equation of the reaction which takes place on adding water to slid X.
(e) Which characteristic of chemical reactions is illustrated by this example?
When metal X is treated with a dilute acid Y, then a gas Z is evolved which burns readily by making a little explosion.
(a) Name any two metals which can behave like metal X.
(b) Name any two acids which can behave like acid Y.
(c) Name the gas Z.
(d) Is the gas Z lighter than or heavier than air?
(e) Is the reaction between metal X and dilute acid Y, exothermic or endothermic?
(f) By taking a specific example of metal X and dilute acid Y, write a balanced chemical equation for the reaction which takes place. Also indicate physical states of all the reactants and products.
A solid substance P which is very hard is used in the construction of many buildings, especially flooring. When substance P is heated strongly, it decomposes to form another solid Q and a gas R is given out. Solid Q reacts with water with the release of a lot of heat to form a substance S. When gas R is passed into a clear solution of substance S, then a white precipitate of substance T is formed. The substance T has the same chemical composition as starting substance P.
(a) What is substance P? Write its common name as well as chemical formula.
(b) What is substance Q?
(c) What is gas R?
(d) What is substance S? What is its clear solution known as?
(e) What is substance T? Name any two natural forms in which substance T occurs in nature.
A silvery-white metal X taken in the form of ribbon, when ignited, burns in air with a dazzling white flame to form a white powder Y. When water is added to powder Y, it dissolves partially to form another substance Z.
(a) What could metal X be?
(b) What is powder Y?
(c) With which substance metal X combines to form powder Y?
(d) What is substance Z? Name one domestic use of substance Z.
(e) Write a balanced chemical equation of the reaction which takes place when metal X burns in air to form powder Y.
A metal X forms a salt XSO4. The salt XSO4 forms a clear solution in water which reacts with sodium hydroxide solution to form a blue precipitate Y. Metal X is used in making electric wire and alloys like brass.
(a) What do you think metal X could be?
(b) Write the name, formula and colour of salt XSO4.
(c) What is the blue precipitate Y?
(d) Write a chemical equation of the reaction which takes place when salt XSO4 reacts with sodium hydroxide solution. Give the state symbols of all the reactants and products which occur in the above equation.
The metal M reacts vigorously with water to form a solution S and a gas G. The solution S turns red litmus to blue whereas gas G, which is lighter than air, burns with a pop sound. Metal M has a low melting point and it is used as a coolant in nuclear reactors.
(a) What is metal M?
(b) What is solution S? Is it acidic or alkaline?
(c) What is gas G?
(d) Write a balanced chemical equation for the reaction which takes place when metal M reacts with water.
(e) Is this reaction exothermic or endothermic?
When a mixture of gases X and Y is compressed to 300 atm pressure and then passed over a catalyst consisting of a mixture of zinc oxide and chromium oxide (heated to a temperature of 300°C), then an organic compounds Z having the molecular formula CH4O is formed. X is a highly poisonous gas which is formed in appreciable amounts when a fuel burns in a limited supply of air; Y is a gas which can be made by the action of a dilute acid on an active metal; and Z is a liquid organic compound which can react with sodium metal to produce hydrogen gas.
(a) What are X, Y and Z?
(b) Write a balanced chemical equation of the reaction which takes place when X and Y combine to form Z. Indicate the conditions under which the reaction occurs.
The white solid compound A decomposes quite rapidly on heating in the presence of a black substance X to form a solid compound B and a gas C. When an aqueous solution of compound B is reacted with silver nitrate solution, then a white precipitate of silver chloride is obtained along with potassium nitrate solution. Gas C does not burn itself but helps burn other things.
(a) What is compound A?
(b) What is compound B?
(c) What is gas C?
(d) What do you think is the black substance X? What is its function?
(e) What is the general name of substances like X?
Gas A, which is the major cause of global warming, combines with hydrogen oxide B in nature in the presence of an environmental factor C and a green material D to form a six carbon organic compounds E and a gas F. The gas F is necessary for breathing.
(a) What is gas A?
(b) What is the common name of B?
(c) What do you think could be C?
(d) What is material D? Where is it found?
(e) Name the organic compound E.
(f) What is gas F? Name the natural process during which it is released.
Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 1 Chemical Reactions and Equations Exercise 2 [Pages 45 - 50]
What type of reaction is represented by the digestion of food in our body?
Name the various types of chemical reactions.
Why does the colour of copper sulphate solution change when an iron nail is kept immersed in it?
Write the balanced chemical equation for the following reaction.
\[\ce{Zinc + Silver nitrate -> Zinc nitrate + Silver}\]
Which term is used to indicate the development of unpleasant smell and taste in fat and oil containing foods due to aerial oxidation (when they are kept exposed for a considerable time)?
What is the general name of the chemicals which are added to fat and oil containing foods to prevent the development of rancidity?
State an important use of decomposition reactions.
What are anti-oxidants? Why are they added to fat and oil containing foods?
Explain why, food products containing fats and oils (like potato chips) are packaged in nitrogen.
Give one example of a decomposition reaction which is carried out with electricity.
Give one example of a decomposition reaction which is carried out by applying heat.
What type of chemical reaction is used to extract metals from their naturally occurring compounds like oxides or chlorides?
Name two anti-oxidants which are usually added to fat and oil containing foods to prevent rancidity.
Write one equation for decomposition reactions where energy is supplied in the form of heat.
In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.
What type of reaction is represented by the following equation?
CaCO3 → CaO + CO2
What type of reaction is represented by the following equation?
CaO + H2O → Ca(OH)
What type of reaction is represented by the following equation?
2 FeSO4 → Fe2 O3 + SO2 + SO3
What type of reaction is represented by the following equation?
NH4 CI → NH3 + HCI
What type of reaction is represented by the following equation?
2 Ca + O2 → 2CaO
What type of chemical reaction take place when a magnesium wire is burnt in air?
What type of chemical reaction take place when lime-stone is heated?
What type of chemical reaction take place when silver bromide is exposed to sunlight?
What type of chemical reaction take place when electricity is passed through water?
What type of chemical reaction take place when ammonia and hydrogen chloride are mixed?
What type of chemical reaction is represented by the following equation?
A + BC → AC + B
What type of chemical reaction is represented by the following equation?
A + B → C
What type of chemical reaction is represented by the following equation?
X → Y + Z
What type of chemical reaction is represented by the following equation?
PQ + RS → PS + RQ
What type of chemical reaction is represented by the following equation?
A2O3 + 2B → B2O3 + 2A
Balance the following chemical equation:

Balance the following chemical equation:
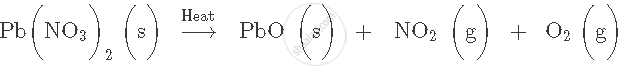
what type of reaction is the following:
Cl2 + 2KI → 2KCl + I2
what tpye of reaction is the following:
2K + Cl → 2KCl
What type of reaction is represented by the following equation?
CaO + CO2 → CaCO3
What type of reaction is represented by the following equation?
2Na + 2H2O → 2NaOH + H2
What type of reaction is represented by the following equation?
Mg + CuSO4 → MgSO4 + Cu
What type of reaction is represented by the following equation?
NH4NO2 → N2 + 2H2O
What type of reaction is represented by the following equation?
CuSO4 + 2NaOH → Cu(OH)2 + Na2SO4
In the following reaction between lead sulphide and hydrogen peroxide:
PbS (s) + 4H2O2(aq) → PbSO4(s) + 4H2O(1)
(a) which substance is reduced?
(b) Which substance is oxidised?
Identify the component oxidised in the following reaction:
H2S + Cl2 → S + 2HCl
When SO2 gas is passed through saturated solution of H2S, the following reaction occurs:
SO2 + 2H2S → 2H2O + 3S
In this reaction, which substance is oxidised and which one is reduced?
Fill in the following blanks with suitable words:
The addition of oxygen to a substance is called ......... whereas removal of oxygen is called ........
Fill in the following blanks with suitable words:
The addition of hydrogen to a substance is called ........ whereas removal of hydrogen is called .......
Fill in the following blank with suitable word:
Anti-oxidants are often added to fat containing foods to prevent .............. due to oxidation.
What is an oxidation reaction? Identify in the following reaction (i) the substance oxidised, and (ii) the substance reduced:
ZnO + C → Zn + CO
Define ‘redox reaction’. Give one example.
When a magnesium ribbon burns in air with a dazzling flame and forms a white ash, is magnesium oxidised or reduced? Why?
In the reaction represented by the equation:
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
(i) name the substance oxidised.
(ii) name the oxidising agent.
(iii) name the substance reduced.
(iv) name the reducing agent.
Define a combination reaction.
Give one example of a combination reaction which is also exothermic.
Give one example of a combination reaction which is also endothermic.
Give an example of an oxidation reaction.
Is oxidation an exothermic or an endothermic reaction?
Explain, by giving an example, how oxidation and reduction proceed side by side.
What is the colour of ferrous sulphate crystals? How does this colour change after heating?
Name the product formed on strongly heating ferrous sulphate crystals. What type of chemical reaction occurs in this change?
What is a decomposition reaction?
Give an example of a decomposition reaction. Describe an activity to illustrate such a reaction by heating.
Zinc oxide reacts with carbon, on heating, to form zinc metal and carbon monoxide. Write a balanced chemical equation for this reaction. Name (i) oxidising agent, (ii) reducing agent, in this reaction.
Give one example of an oxidation-reduction reaction which is also a combination reaction.
Give one example of an oxidation-reduction reaction which is also a displacement reaction.
What is the difference between displacement and double displacement reactions? Write equations for these reactions.
What do you mean by a precipitation reaction? Explain by giving examples.
Explain oxidation in terms of gain or loss of oxygen with one example.
Explain reduction in terms of gain or loss of oxygen with one example.
When copper powder is heated strongly in air, it forms copper oxide. Write a balanced chemical equation for this reaction. Name (i) substance oxidised, and (ii) substance reduced.
Define oxidation in terms of gain or loss of hydrogen with one example.
Define reduction in terms of gain or loss of hydrogen with one example.
When a magnesium ribbon is heated, it burns in air to form magnesium oxide. Write a balanced chemical equation for this reaction. Name (i) substance oxidised, and (ii) substance reduced.
What is meant by displacement reaction, Explain with help of one example.
What is meant by double displacement reaction? Explain with help of one example.
Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Express the following facts in the form of a balanced chemical equation:
"When a strip of copper metal is placed in a solution of silver nitrate, metallic silver is precipitated and a solution containing copper nitrate is formed".
What happens when a piece of iron metal is placed in copper sulphate solution? Name the type of reaction involved.
Write balanced chemical equation with state symbols for the following reaction:
Barium chloride solution reacts with sodium sulphate solution to give insoluble barium sulphate and a solution of sodium chloride.
In the reaction represented by the following equation:
CuO (s) + H2 (g) → Cu (s) + H2O (1)
(a) name the substance oxidised
(b) name the substance reduced
(c) name the oxidising agent
(d) name the reducing agent
What happens when silver nitrate solution is added to sodium chloride solution?
Write the equation for the reaction which takes place.
What happens when silver nitrate solution is added to sodium chloride solution?
Name the type of reaction involved.
State the change in colour observed in following case mentioning the reason:
Silver chloride is exposed to sunlight.
What happens when a zinc strip is dipped into a copper sulphate solution?
Write the equation for the reaction that takes place.
What happens when a zinc strip is dipped into a copper sulphate solution?
Name the type of reaction involved.
Explain the terms Corrosion
Write a chemical equation to show the process of corrosion of iron.
What special name is given to their corrosion of iron?
What type of chemical reaction is involved in the corrosion of iron?
Name any three objects (or structures) which are gradually damaged by the corrosion of iron and steel.
Explain the term Rancidity.
What damage is caused by rancidity?
What type of chemical reaction is responsible for causing rancidity?
State and explain the various methods for preventing or retarding rancidity of food.
(a) What happens when an aqueous solution of sodium sulphate reacts with an aqueous solution of barium chloride?
(b) Write the balanced chemical equation for the reaction which takes place.
(c) State the physical conditions of reactants in which the reaction will not take place.
(d) Name the type of chemical reaction which occurs.
(e) Give one example of another reaction which is of the same type as the above reaction.
The removal of oxygen from a substance is called:
(a) oxidation
(b) corrosion
(c) reduction
(d) rancidity
In the context of redox reactions, the removal of hydrogen from a substance is known as:
(a) oxidation
(b) dehydration
(c) reduction
(d) dehydrogenation
The chemical reaction involved in the corrosion of iron metal is that of:
(a) oxidation as well as displacement
(b) reduction as well as combination
(c) oxidation as well as combination
(d) reduction as well as displacement
The term used to indicate the development of unpleasant smell and taste in fat and oil containing foods due to aerial oxidation is:
(a) acidity
(b) radioactivity
(c) rabidity
(d) rancidity
In order to prevent the spoilage of potato chips, they are packed in plastic bags in an atmosphere of:
(a) Cl2
(b) H2
(c) N2
(d) O2
A white precipitate can be obtained by adding dilute sulphuric acid to:
(a) CuSO4 solution
(b) Nacl solution
(c) BaCl2 solution
(d) Na2SO4 solution
A white precipitate will be formed if we add common salt solution to:
(a) Ba(NO3)2 solution
(b) KNO3 solution
(c) AgNO3 solution
(d) Mg(NO3)2 solution
Consider the following equation of the chemical reaction of a metal M:
4M + 3O2 → 2M2O3
This equation represents:
(a) combination reaction as well as reduction reaction
(b) decomposition reaction as well as oxidation reaction
(c) oxidation reaction as well as displacement reaction
(d) combination reaction as well as oxidation reaction
The process of respiration is:
(a) an oxidation reaction which is endothermic
(b) a reduction reaction which is exothermic
(c) a combination reaction which is endothermic
(d) an oxidation reaction which is exothermic
Which of the following can be decomposed by the action of light?
(a) NaCl
(b) KCl
(c) AgCl
(d) CuCl
Consider the reaction:
KBr (aq) + AgNO3 (aq) → KNO3 (aq) + AgBr (s)
This is an example of:
(a) decomposition reaction
(b) combination reaction
(c) double displacement reaction
(d) displacement reaction
You are given the following chemical equation:
Mg (s) + CuO (s) → MgO (s) + Cu (s)
This equation represents:
(a) decomposition reaction as well as displacement reaction
(b) combination reaction as well as double displacement reaction
(c) redox reaction as well as displacement reaction
(d) double displacement reaction as well as redox reaction
When a green iron salt is heated strongly, its colour finally changes to brown and odour of burning sulphur is given out.
(a) Name the iron salt.
(b) Name the type of reaction that takes place during the heating of iron salt.
(c) Write a chemical equation for the reaction involved.
A colourless lead salt, when heated, produces a yellow residue and brown fumes.
(a) Name the lead salt.
(b) Name the brown fumes.
(c) Write a chemical equation of the reaction involved.
When hydrogen burns in oxygen, water is formed and when water is electrolysed, then hydrogen and oxygen are produced. What type of reaction takes place:
(a) in the first case?
(b) in the second case
A strip of metal X is dipped in a blue coloured salt solution YSO4. After some time, a layer of metal Y from the salt solution is formed on the surface of metal strip X. Metal X is used in galvanisation whereas metal Y is used in making electric wires. Metal X and metal Y together form an alloy Z.
(a) What could metal X be?
(b) What could metal Y be?
(c) Name the metal salt YSO4.
(d) What type of chemical reaction takes place when metal X reacts with salt solution YSO4? Write the equation of the chemical reaction involved.
(e) Name the alloy Z.
When a black metal compound XO is heated with a colourless gas Y2, then metal X and another compound Y2O are formed. Metal X is red-brown in colour which does not react with dilute acids at all. Gas Y2 can be prepared by the action of a dilute acid on any active metal. The compound Y2O is a liquid at room temperature which can turn anhydrous copper sulphate blue.
(a) What do you think is metal X?
(b) What could be gas Y2?
(c) What is compound XO?
(d) What is compound Y2O?
(e) Write the chemical equation of the reaction which takes place on heating XO with Y2.
(f) What type of chemical reaction is illustrated in the above equation?
A metal X forms a water soluble salt XNO3. When an aqueous solution of XNO3 is added to common salt solution, then a white precipitate of compound Y is formed alongwith sodium nitrate solution. Metal X is said to be the best conductor of electricity and it does not evolve hydrogen when put in dilute hydrohloric acid.
(a) What is metal X?
(b) What is salt XNO3?
(c) Name the compound Y.
(d) Write the chemical equation of the reaction which takes place on reacting XNO3 solution and common salt solution giving the physical states of all the reactants and products.
(e) What type of chemical reaction is illustrated by the above equation?
Two metals X and Y form the salts XSO4 and Y2SO4, respectively. The solution of salt XSO4 is blue in colour whereas that of Y2SO4 is colourless. When barium chloride solution is added to XSO4 solution, then a white precipitate Z is formed alongwith a salt which turns the solution green. And when barium chloride solution is added to Y2SO4 solution, then the same white precipitate Z is formed alongwith colourless common salt solution.
(a) What could the metals X and Y be?
(b) Write the name and formula of salt XSO4.
(c) Write the name and formula of salt Y2SO4.
(d) What is the name and formula of white precipitate Z?
(e) Write the name and formula of the salt which turns the solution green in the first case.
A red-brown metal X forms a salt XSO4. When hydrogen sulphide gas is passed through an aqueous solution of XSO4, then a black precipitate of XS is formed alongwith sulphuric acid solution.
(a) What could the salt XSO4 be?
(b) What is the colour of salt XSO4?
(c) Name the black precipitate XS.
(d) By using the formula of the salt obtained in (a) above, write an equation of the reaction which takes place when hydrogen sulphide gas is passed through its aqueous solution.
(e) What type of chemical reaction takes place in this case?
When a strip of red-brown metal X is placed in a colourless salt solution YNO3 then metal Y is set free and a blue coloured salt solution X(NO3)2 is formed. The liberated metal Y forms a shining white deposit on the strip of metal X.
(a) What do you think metal X is?
(b) Name the salt YNO3.
(c) What could be metal Y?
(d) Name the salt X(NO3)2.
(e) What type of reaction takes place between metal X and salt solution YNO3?
A metal salt MX when exposed to light splits up to form metal M and a gas X2. Metal M is used in making ornaments whereas gas X2 is used in making bleaching powder. The salt MX is itself used in black and white photography.
(a) What do you think metal M is?
(b) What could be gas X2?
(c) Name the metal salt MX.
(d) Name any two salt solutions which on mixing together can produce a precipitate of salt MX.
(e) What type of chemical reaction takes place when salt MX is exposed to light? Write the equation of the reaction?
Solutions for 1: Chemical Reactions and Equations
![Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 chapter 1 - Chemical Reactions and Equations Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 chapter 1 - Chemical Reactions and Equations - Shaalaa.com](/images/9352831810-chemistry-science-english-class-10_6:7eeaf5db0f4a49c580d3c8746ff5ab36.jpg)
Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 chapter 1 - Chemical Reactions and Equations
Shaalaa.com has the CBSE Mathematics Chemistry (Science) [English] Class 10 CBSE solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Lakhmir Singh solutions for Mathematics Chemistry (Science) [English] Class 10 CBSE 1 (Chemical Reactions and Equations) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Lakhmir Singh textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry (Science) [English] Class 10 chapter 1 Chemical Reactions and Equations are Chemical Equation, Direct Combination (or Synthesis) Reaction, Decomposition Reactions, Types of Chemical Change or Chemical Reaction, Balancing Chemical Equation, Single Displacement Reactions, Double Displacement Reaction, Oxidation, Reduction and Redox Reactions, Corrosion of Metals, Rancidity of Food and Its Prevention.
Using Lakhmir Singh Chemistry (Science) [English] Class 10 solutions Chemical Reactions and Equations exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Lakhmir Singh Solutions are essential questions that can be asked in the final exam. Maximum CBSE Chemistry (Science) [English] Class 10 students prefer Lakhmir Singh Textbook Solutions to score more in exams.
Get the free view of Chapter 1, Chemical Reactions and Equations Chemistry (Science) [English] Class 10 additional questions for Mathematics Chemistry (Science) [English] Class 10 CBSE, and you can use Shaalaa.com to keep it handy for your exam preparation.
