Advertisements
Advertisements
Question
State one characteristic of the chemical reaction which takes place when dilute sulphuric acid is added to barium chloride solution.
Solution
When dilute sulphuric acid is added to barium chloride solution, it forms white precipitates of barium sulphate. Hence, this reaction is characterised by formation of precipitate.
APPEARS IN
RELATED QUESTIONS
Write the balanced chemical equation for the following reaction.
\[\ce{Zinc + Silver nitrate -> Zinc nitrate + Silver}\]
Balance the given equation:
BaCI2 + H2SO4  BaSO4 + HCI
BaSO4 + HCI
When hydrogen is passed over copper oxide, copper and steam are formed. Write a balanced equation for this reaction and state which of the chemicals are metals.
Balance the following chemical equation:
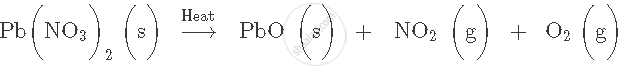
Write word equation for the following molecular equation:
CuCo3 → CuO + CO2 [g]
Word equation:
State the characteristics or indications occurring in the above reaction.
In certain reaction an insoluble solid called precipitate is formed. State the colour and name of the precipitate formed in the following reaction involving addition of:
Dilute hydrochloric acid to silver nitrate.
Representation of the results of a chemical change – is a chemical equation.
For the equation: FeCl3 + 3NH4OH 3NH4Cl + Fe(OH)3 ↓
Answer the following:
Name the reactants and the products in the above equation.
Give an example of a chemical equation in which two reactants form:
four product
CaCO3 + 2HCl[dil.] → CaCl2 + H2O + CO2 [g]
State the information provided by the above chemical equation.
Complete the following blank in the equation as indicated.
\[\ce{CaH2_{(s)} + 2H2O_{( aq)}-> Ca(OH)2_{(s)} + 2H2_{(g)}}\]
Molecules: 6.02 × 1023 + ______ `→` ______ + ______
