Advertisements
Advertisements
Question
State one characteristic of the chemical reaction which takes place when lemon juice is added gradually to potassium permanganate solution.
Solution
Lemon juice (contains citric acid) changes the purple colour of potassium permanganate solution to a colourless solution. Hence, this reaction is characterised by change in colour.
APPEARS IN
RELATED QUESTIONS
When hydrogen is passed over copper oxide, copper and steam are formed. Write a balanced equation for this reaction and state which of the chemicals are elements.
The chemical reaction between quicklime and water is characterised by:
(a) evolution of hydrogen gas
(b) formation of slaked lime precipitate
(c) change in temperature of mixture
(d) change in colour of the product
Balance the following chemical equation:
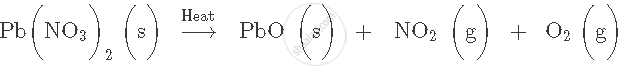
Balance the following equation. Also name the products formed.
\[\ce{Ag + S -> Ag2S}\]
Balance the following equation:
MnO2 + HCl → MnCl2 + H2O + Cl2
Write the balanced chemical equation of the following reaction.
iron pyrites(FeS2) + oxygen → ferric oxide + sulphur dioxide
Name the substances that are getting oxidised and reduced in the process.
In certain reaction an insoluble solid called precipitate is formed. State the colour and name of the precipitate formed in the following reaction involving addition of:
Iron [II] sulphate to sodium hydroxide.
Give one example in the case where supplying energy [given below] is necessary for a chemical reaction.
Heat energy
Give one example in the case where supplying energy [given below] is necessary for a chemical reaction.
Catalyst
